Introduction
The initial evaluation of a patient with suspected kidney disease is a critical step in diagnosing and managing a wide spectrum of renal pathologies. A systematic approach, integrating clinical history, physical examination, and targeted laboratory and imaging investigations, is essential for accurate diagnosis, prognostication, and the development of an effective management plan. This chapter outlines a comprehensive framework for the assessment of kidney disease, covering the interpretation of signs and symptoms, the evaluation of kidney function, the role of urinalysis, and the application of various imaging and diagnostic tests.
Signs and Symptoms of Kidney Disease
Chronic kidney disease (CKD) is often a silent disease in its early stages, with many patients remaining asymptomatic until significant renal function has been lost. The clinical presentation of kidney disease varies widely depending on the underlying etiology, the severity of renal dysfunction, and the presence of comorbidities. Early recognition of the subtle signs and symptoms is crucial for timely intervention and to slow the progression of the disease.
Early vs. Late-Stage Presentation
In the early stages of CKD (stages 1-3), patients are typically asymptomatic, and the disease is often detected incidentally through routine laboratory testing. As the disease progresses to more advanced stages (stages 4-5), a constellation of signs and symptoms, collectively known as uremia, may develop. These clinical manifestations are a direct consequence of the accumulation of metabolic waste products, electrolyte and acid-base imbalances, and the dysregulation of various hormonal and metabolic functions of the kidneys.
| Stage of CKD | Typical Presentation | Common Signs and Symptoms |
| Early (Stages 1-3) | Often asymptomatic | Abnormal laboratory findings (e.g., proteinuria, elevated creatinine) without overt symptoms. |
| Late (Stages 4-5) | Symptomatic (Uremia) | Nausea, vomiting, loss of appetite, fatigue, weakness, sleep disturbances, oliguria, decreased mental sharpness, muscle cramps, edema, pruritus, chest pain, shortness of breath, and hypertension. 1 |
Physical Examination Findings
A thorough physical examination can provide valuable clues to the presence and chronicity of kidney disease. While many findings are non-specific, certain signs are highly suggestive of advanced renal dysfunction.
Physical findings such as skin pigmentation, scratch marks, left ventricular hypertrophy, and hypertensive fundal changes suggest chronicity. 1
Key physical examination findings in patients with advanced CKD may include:
•General: Cachexia, pallor (due to anemia).
•Skin: Dry, itchy skin (pruritus), uremic frost (a rare finding of crystallized urea on the skin), skin pigmentation, and scratch marks.
•Cardiovascular: Hypertension, signs of fluid overload (e.g., peripheral edema, pulmonary rales, elevated jugular venous pressure), and a pericardial friction rub (suggesting uremic pericarditis).
•Neurological: Decreased mental sharpness, confusion, asterixis (flapping tremor), myoclonus, and hyperreflexia.
•Ophthalmologic: Hypertensive retinopathy (fundal changes).
Assessment of Kidney Function
The cornerstone of assessing kidney function is the estimation of the glomerular filtration rate (GFR), which represents the volume of fluid filtered from the renal glomerular capillaries into the Bowman’s capsule per unit time. GFR is the best overall index of kidney function and is central to the detection, diagnosis, and staging of kidney disease.
Glomerular Filtration Rate (GFR)
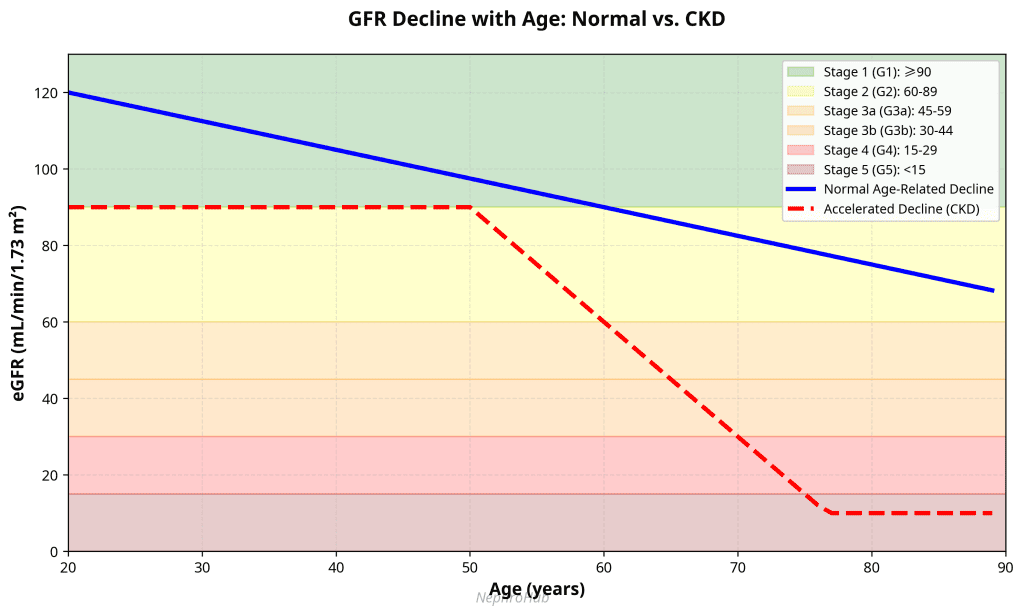
A normal GFR in a young adult male is approximately 90 to 120 mL/min/1.73 m². GFR naturally declines with age, at an average rate of about 0.75 to 1 mL/min/year after the age of 40 to 50. 2 The ideal marker for measuring GFR would be a substance that is endogenously produced at a constant rate, freely filtered at the glomerulus, and neither reabsorbed nor secreted by the renal tubules. While no perfect endogenous marker exists, several methods are used to estimate GFR in clinical practice.
Creatinine and Creatinine Clearance
Creatinine, a by-product of muscle metabolism, is the most commonly used endogenous marker for estimating GFR. It is produced at a relatively constant rate and is primarily cleared by the kidneys. A decrease in GFR leads to an increase in serum creatinine levels. However, serum creatinine alone is an imperfect marker of GFR due to its dependence on muscle mass, age, sex, and diet. Furthermore, a significant decrease in GFR (up to 50%) can occur before serum creatinine levels rise above the normal range. 2
Creatinine clearance, calculated from a timed urine collection (usually 24 hours) and a simultaneous plasma creatinine measurement, provides a more accurate estimate of GFR than serum creatinine alone. The formula for creatinine clearance is:
C = (Ucr x V) / Pcr
Where:
•C = Clearance
•Ucr = Urinary creatinine concentration
•V = Urine flow rate
•Pcr = Plasma creatinine concentration
Despite its utility, creatinine clearance is prone to errors, primarily due to incomplete or inaccurate urine collection. Additionally, because creatinine is actively secreted by the renal tubules, creatinine clearance tends to overestimate the true GFR by 10-20%. 2
Estimated GFR (eGFR) Equations
To overcome the limitations of serum creatinine and timed urine collections, several equations have been developed to estimate GFR from serum creatinine, taking into account variables such as age, sex, and race. The most widely used and recommended equation is the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation. 2 This equation is more accurate than the previously used Modification of Diet in Renal Disease (MDRD) equation, especially at higher GFR levels.
Other markers, such as cystatin C, are also used to estimate GFR. Cystatin C is a protein produced by all nucleated cells and is less dependent on muscle mass than creatinine. Equations combining both creatinine and cystatin C can provide an even more accurate estimation of GFR. 2
Staging of Chronic Kidney Disease
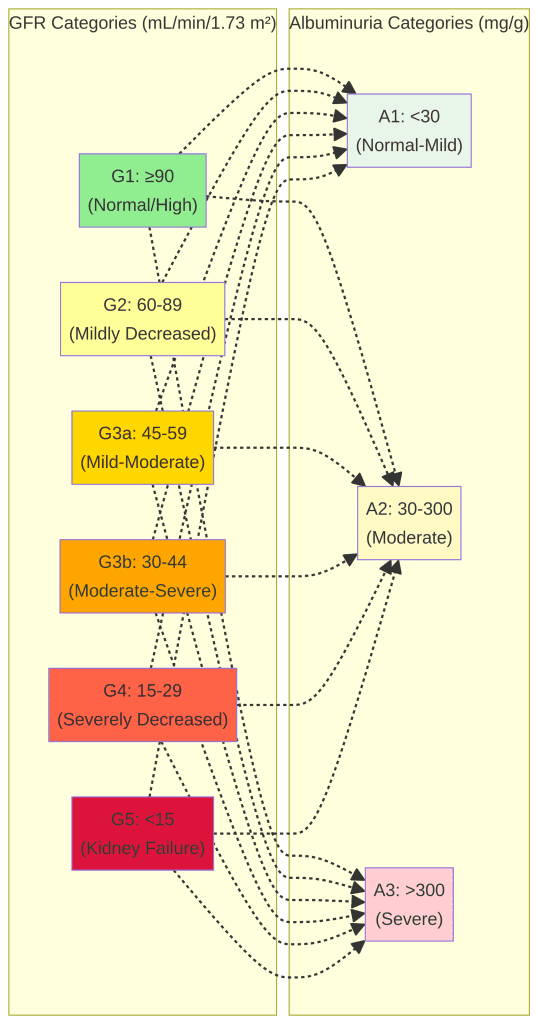
CKD is staged based on the GFR and the level of albuminuria, as defined by the Kidney Disease: Improving Global Outcomes (KDIGO) guidelines. This staging system helps to classify the severity of CKD, predict the risk of progression and complications, and guide management decisions.
| GFR Stage | GFR (mL/min/1.73 m²) | Description |
| G1 | ≥90 | Normal GFR with evidence of kidney damage |
| G2 | 60-89 | Mildly decreased GFR with evidence of kidney damage |
| G3a | 45-59 | Mildly to moderately decreased GFR |
| G3b | 30-44 | Moderately to severely decreased GFR |
| G4 | 15-29 | Severely decreased GFR |
| G5 | <15 | Kidney failure (or on dialysis) |
| Albuminuria Stage | Albumin-to-Creatinine Ratio (ACR) | Description |
| A1 | <30 mg/g | Normal to mildly increased |
| A2 | 30-300 mg/g | Moderately increased |
| A3 | >300 mg/g | Severely increased |
Urine Analysis
Urinalysis is a simple, non-invasive, and highly informative test that is fundamental to the evaluation of kidney disease. It involves a physical, chemical, and microscopic examination of the urine, providing valuable insights into the underlying renal pathology.
Components of a Urinalysis
A complete urinalysis consists of three main components:
1.Physical Examination: Assessment of the urine’s color, clarity, and specific gravity.
2.Chemical Examination: A dipstick test that screens for pH, protein, glucose, ketones, blood, leukocyte esterase, nitrites, bilirubin, and urobilinogen.
3.Microscopic Examination: Microscopic analysis of the urine sediment to identify cells, casts, crystals, and microorganisms.
Microscopic Examination of Urine Sediment
The microscopic examination of urine sediment is a crucial part of the urinalysis, as it can help to localize the site of injury within the kidney and narrow the differential diagnosis. Key findings in the urine sediment include:
•Cells: The presence of red blood cells (RBCs), white blood cells (WBCs), and renal tubular epithelial cells can indicate various forms of kidney disease. Dysmorphic (abnormally shaped) RBCs are particularly suggestive of glomerular disease. 3
•Casts: Casts are cylindrical structures formed in the renal tubules from a matrix of Tamm-Horsfall protein. The type of cast is determined by the cellular elements trapped within it. For example, RBC casts are a hallmark of glomerulonephritis, while WBC casts are characteristic of pyelonephritis or interstitial nephritis. 3
•Crystals: While some crystals can be found in the urine of healthy individuals, certain types of crystals are associated with specific pathological conditions. For instance, cystine crystals are diagnostic of cystinuria. 3
| Microscopic Finding | Clinical Significance |
| Dysmorphic RBCs | Glomerular disease |
| RBC Casts | Glomerulonephritis, vasculitis |
| WBC Casts | Pyelonephritis, interstitial nephritis |
| Renal Tubular Epithelial Cell Casts | Acute tubular necrosis |
| Fatty Casts | Nephrotic syndrome |
| Waxy/Broad Casts | Advanced chronic kidney disease |
Imaging of the Kidneys
Renal imaging plays a vital role in the evaluation of kidney disease, providing anatomical and, in some cases, functional information. The choice of imaging modality depends on the clinical question, patient characteristics, and local availability.
Ultrasound
Renal ultrasound is the most commonly used initial imaging modality in nephrology. It is non-invasive, widely available, and does not involve ionizing radiation or iodinated contrast. Ultrasound is excellent for assessing kidney size, cortical thickness, and echogenicity, which can help to distinguish between acute and chronic kidney disease. It is also the primary modality for detecting hydronephrosis (swelling of the kidneys due to urine backup) and evaluating for urinary tract obstruction. 4
Computed Tomography (CT)
CT provides detailed anatomical images of the kidneys and surrounding structures. Non-contrast CT is the gold standard for detecting kidney stones. Contrast-enhanced CT is useful for evaluating renal masses, complex cysts, and renal trauma. CT angiography (CTA) is a highly accurate method for visualizing the renal arteries and diagnosing renal artery stenosis. However, the use of iodinated contrast agents carries a risk of contrast-induced nephropathy, particularly in patients with pre-existing CKD. 4
Magnetic Resonance Imaging (MRI)
MRI offers excellent soft-tissue contrast without the use of ionizing radiation. It is particularly useful for characterizing renal masses and can be used as an alternative to CT in patients with contraindications to iodinated contrast. MR angiography (MRA) can be performed with or without gadolinium contrast to evaluate the renal vasculature. However, the use of gadolinium-based contrast agents is contraindicated in patients with severe CKD (eGFR < 30 mL/min/1.73 m²) due to the risk of nephrogenic systemic fibrosis (NSF), a rare but serious fibrosing disorder. 4
Nuclear Medicine (Renal Scintigraphy)
Renal scintigraphy is a functional imaging technique that uses radiopharmaceuticals to assess various aspects of kidney function, including perfusion, filtration, and excretion. It is used to determine differential (split) renal function, evaluate for urinary tract obstruction, and diagnose renovascular hypertension (captopril renography). 4
Other Diagnostic Tests
In addition to the tests described above, a variety of other diagnostic tests may be employed in the evaluation of kidney disease, depending on the clinical context.
Blood Tests
A comprehensive metabolic panel, including electrolytes, blood urea nitrogen (BUN), and glucose, is essential. A complete blood count (CBC) can reveal anemia, a common complication of CKD. Other relevant blood tests include measurements of calcium, phosphorus, and parathyroid hormone (PTH) to assess for mineral and bone disorders associated with CKD.
Kidney Biopsy
A percutaneous kidney biopsy is the gold standard for diagnosing many types of kidney disease, particularly glomerulonephritis and other parenchymal diseases. It provides a definitive histological diagnosis, which can guide treatment and provide prognostic information.
Summary of the Clinical Approach
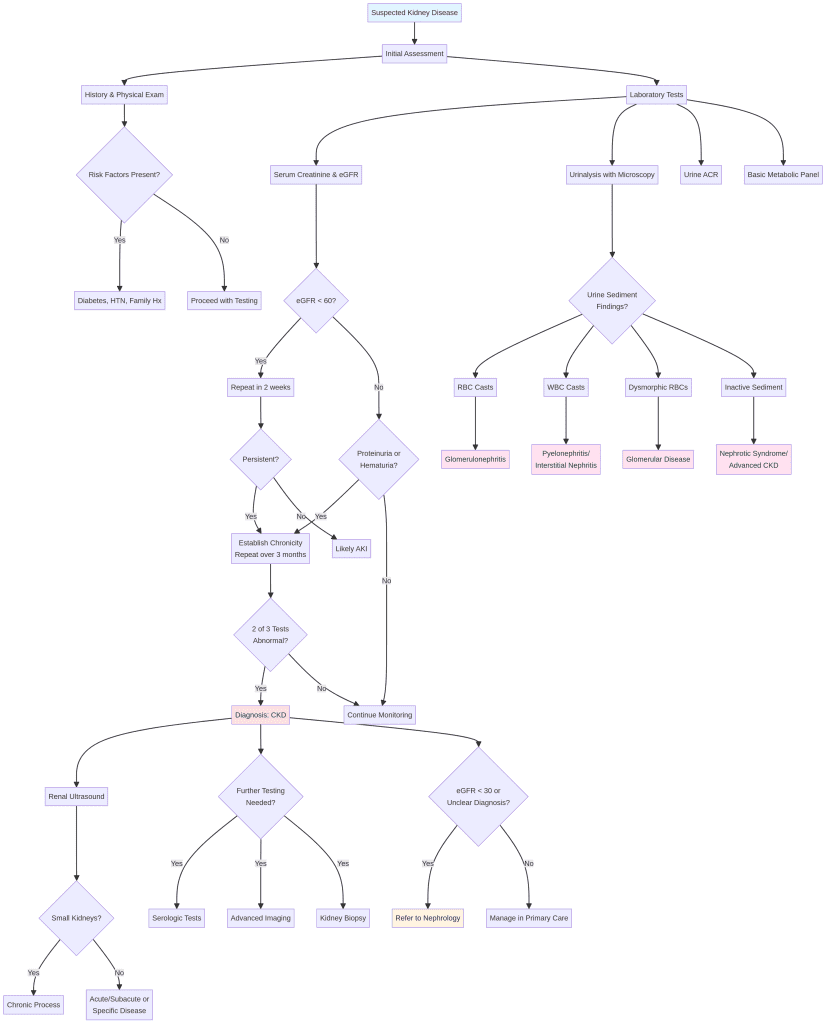
The evaluation of a patient with suspected kidney disease should follow a logical, stepwise approach:
1.History and Physical Examination: Gather a detailed history and perform a thorough physical examination to identify risk factors, symptoms, and signs of kidney disease.
2.Initial Laboratory Tests: Obtain a serum creatinine to calculate the eGFR, a complete urinalysis with microscopic examination, and a urine albumin-to-creatinine ratio (ACR).
3.Initial Imaging: Perform a renal ultrasound to assess kidney size and structure and to rule out obstruction.
4.Establish Chronicity: If abnormalities are found, repeat testing over a three-month period to confirm the diagnosis of CKD.
5.Further Investigation: Based on the initial findings, consider further investigations, such as serological testing for specific diseases, advanced imaging, or a kidney biopsy, to determine the underlying cause of the kidney disease.
By following this systematic approach, clinicians can effectively diagnose and manage kidney disease, with the ultimate goal of preserving renal function and improving patient outcomes.
Summary
Key Points
Signs and Symptoms:
•Early-stage CKD (stages 1-3) is typically asymptomatic and detected through routine laboratory screening. Advanced CKD (stages 4-5) presents with uremic symptoms including nausea, fatigue, pruritus, edema, and altered mental status.
•Physical examination findings suggesting chronicity include skin pigmentation, scratch marks, hypertensive retinopathy, and signs of fluid overload.
Assessment of Kidney Function:
•GFR is the best overall indicator of kidney function. Normal GFR is 90-120 mL/min/1.73 m² in young adults and declines with age at approximately 0.75-1 mL/min/year after age 40-50.
•Serum creatinine is influenced by muscle mass, age, sex, and diet. A 50% reduction in GFR can occur before creatinine rises above normal range.
•The CKD-EPI equation is the most widely recommended method for estimating GFR from serum creatinine.
•CKD is staged using both GFR categories (G1-G5) and albuminuria categories (A1-A3) according to KDIGO guidelines.
Urinalysis:
•A complete urinalysis includes physical, chemical (dipstick), and microscopic examination of urine.
•Microscopic findings provide crucial diagnostic information: dysmorphic RBCs and RBC casts suggest glomerular disease, WBC casts indicate pyelonephritis or interstitial nephritis, and renal tubular epithelial cell casts suggest acute tubular necrosis.
•The presence and type of urinary casts help localize the site of kidney injury.
Imaging:
•Renal ultrasound is the first-line imaging modality, providing information on kidney size, cortical thickness, echogenicity, and the presence of obstruction without radiation or contrast exposure.
•CT is excellent for detecting kidney stones (non-contrast) and evaluating renal masses, but carries radiation risk and potential for contrast-induced nephropathy.
•MRI offers superior soft-tissue contrast without radiation but is contraindicated in severe CKD when gadolinium contrast is needed due to NSF risk.
•Nuclear medicine scans provide functional assessment and are useful for determining split renal function and diagnosing renovascular hypertension.
Clinical Approach:
•Initial evaluation should include history, physical examination, serum creatinine with eGFR calculation, complete urinalysis with microscopy, and urine albumin-to-creatinine ratio.
•Renal ultrasound should be performed early to assess kidney structure and rule out obstruction.
•Chronicity is established by repeating abnormal tests over a three-month period, with at least 2 of 3 results being positive.
•Further investigations, including serological tests, advanced imaging, or kidney biopsy, should be guided by initial findings and clinical suspicion.
Clinical Pearls
Diagnostic Pearls
Pearl 1: The “Creatinine Blind Spot”
Serum creatinine is a late marker of kidney dysfunction. Up to 50% of kidney function can be lost before creatinine levels rise above the normal range. Always calculate eGFR and don’t rely on creatinine alone.
Pearl 2: Establishing Chronicity
To distinguish acute kidney injury from chronic kidney disease, look for: (1) previous laboratory results showing elevated creatinine or proteinuria, (2) small kidneys on ultrasound (<9 cm), (3) anemia with elevated PTH, and (4) hypertensive retinopathy. Normal PTH levels favor AKI over CKD.
Pearl 3: The Power of Urine Microscopy
Don’t skip the microscopic examination of urine sediment. RBC casts are pathognomonic for glomerulonephritis, while WBC casts point to pyelonephritis or interstitial nephritis. The presence of these casts can guide your diagnostic workup and avoid unnecessary testing.
Pearl 4: Dysmorphic RBCs vs. Isomorphic RBCs
Dysmorphic (irregularly shaped) RBCs in urine suggest glomerular bleeding, while isomorphic (normal-shaped) RBCs suggest bleeding from the lower urinary tract. This distinction is crucial in narrowing your differential diagnosis.
Pearl 5: Proteinuria Quantification
A spot urine albumin-to-creatinine ratio (ACR) is more practical than a 24-hour urine collection and is the recommended method by KDIGO. An ACR >300 mg/g indicates nephrotic-range proteinuria and warrants further investigation for nephrotic syndrome or diabetic nephropathy.
Management Pearls
Pearl 6: Avoid Nephrotoxins
In patients with CKD, avoid NSAIDs, aminoglycosides, and high-dose contrast agents whenever possible. If contrast is necessary, ensure adequate hydration and consider using iso-osmolar or low-osmolar contrast agents.
Pearl 7: The “Three-Month Rule”
CKD is defined by kidney damage or eGFR <60 mL/min/1.73 m² persisting for at least 3 months. Always repeat abnormal kidney function tests to confirm chronicity before labeling a patient with CKD.
Pearl 8: When to Order a Kidney Biopsy
Consider kidney biopsy when: (1) the cause of kidney disease is unclear, (2) there is unexplained acute kidney injury, (3) nephrotic syndrome is present, (4) rapidly progressive glomerulonephritis is suspected, or (5) the diagnosis will significantly alter management.
Pearl 9: Ultrasound Limitations
While ultrasound is excellent for assessing kidney size and detecting obstruction, it has limited ability to characterize renal masses or evaluate the renal vasculature. If a mass is detected on ultrasound, proceed with CT or MRI for further characterization.
Pearl 10: Gadolinium and Severe CKD
Avoid gadolinium-based contrast agents in patients with eGFR <30 mL/min/1.73 m² due to the risk of nephrogenic systemic fibrosis (NSF). If MRI is needed, use non-contrast sequences or consider alternative imaging modalities.
Interpretation Pearls
Pearl 11: The Significance of Small Kidneys
Small kidneys (<9 cm in length) on ultrasound suggest chronic, irreversible kidney disease. Exceptions include congenital small kidneys and chronic reflux nephropathy. Normal or enlarged kidneys suggest acute or subacute processes, or specific diseases like diabetic nephropathy, amyloidosis, or polycystic kidney disease.
Pearl 12: Urine Dipstick Limitations
Standard urine dipsticks detect albumin but may miss other proteins such as light chains (Bence Jones protein) in multiple myeloma. If myeloma is suspected, order a urine protein electrophoresis.
Pearl 13: The “Active” Urine Sediment
An “active” urine sediment with RBCs, WBCs, and casts suggests an acute glomerular or tubulointerstitial process. An “inactive” sediment with heavy proteinuria suggests nephrotic syndrome or advanced CKD with glomerulosclerosis.
Pearl 14: Captopril Renography
Captopril (ACE inhibitor) renography can help diagnose renovascular hypertension by demonstrating a decrease in GFR in the affected kidney after ACE inhibitor administration. However, it is less sensitive in patients with bilateral renal artery stenosis or baseline eGFR <30 mL/min/1.73 m².
Pearl 15: Annual Screening for High-Risk Patients
Patients with diabetes, hypertension, family history of kidney disease, or cardiovascular disease should undergo annual screening with serum creatinine, eGFR, and urine ACR to detect CKD early and prevent progression.
Quick Reference: Approach to Kidney Disease
Initial Evaluation Checklist
Detailed history (risk factors, medications, family history)
Physical examination (blood pressure, edema, skin changes)
Serum creatinine and eGFR calculation
Complete urinalysis with microscopy
Urine albumin-to-creatinine ratio (ACR)
Basic metabolic panel (electrolytes, BUN, glucose)
Renal ultrasound
Complete blood count (assess for anemia)
Red Flags Requiring Urgent Evaluation
•Rapidly rising creatinine (>0.5 mg/dL increase in 24-48 hours)
•Oliguria or anuria
•Severe hypertension (>180/120 mmHg)
•Pulmonary edema
•Severe hyperkalemia (K+ >6.5 mEq/L)
•Uremic symptoms (pericarditis, encephalopathy, bleeding)
•RBC casts in urine (suggests glomerulonephritis)
When to Refer to Nephrology
•eGFR <30 mL/min/1.73 m² (stage 4-5 CKD)
•Rapidly progressive kidney disease
•Nephrotic syndrome (proteinuria >3.5 g/day + edema + hypoalbuminemia)
•Glomerulonephritis (active urine sediment with RBC casts)
•Unclear diagnosis requiring kidney biopsy
•Difficult-to-control hypertension in CKD
•Electrolyte abnormalities resistant to treatment
•Preparation for renal replacement therapy
Assessment and Diagnosis MCQs
Question 1
A 65-year-old man with type 2 diabetes presents for routine follow-up. His serum creatinine is 1.4 mg/dL (124 μmol/L), which is within the laboratory’s normal range. What is the most appropriate next step?
A. Reassure the patient that his kidney function is normal
B. Calculate the estimated glomerular filtration rate (eGFR)
C. Order a kidney biopsy
D. Start dialysis preparation
Correct Answer: B
Explanation:
Serum creatinine alone is an inadequate marker of kidney function because it can remain within the normal range even when up to 50% of kidney function has been lost. This occurs because creatinine production depends on muscle mass, age, sex, and dietary intake, and the relationship between creatinine and GFR is not linear. In this patient with diabetes (a major risk factor for chronic kidney disease), it is essential to calculate the eGFR using equations such as CKD-EPI that account for age, sex, and race. An eGFR calculation would provide a more accurate assessment of kidney function. A creatinine of 1.4 mg/dL in a 65-year-old man might correspond to an eGFR of 50-60 mL/min/1.73 m², indicating Stage 3 CKD, which would require further evaluation and management.
Key Learning Point: Always calculate eGFR rather than relying on serum creatinine alone, especially in high-risk patients such as those with diabetes, hypertension, or elderly individuals.
Question 2
A 45-year-old woman presents with new-onset edema and frothy urine. Urinalysis shows 4+ protein. Urine microscopy reveals oval fat bodies and fatty casts. What is the most likely diagnosis?
A. Acute tubular necrosis
B. Nephrotic syndrome
C. Urinary tract infection
D. Acute interstitial nephritis
Correct Answer: B
Explanation:
The clinical presentation of edema, heavy proteinuria (4+ on dipstick), and the microscopic finding of oval fat bodies and fatty casts are classic for nephrotic syndrome. Oval fat bodies are renal tubular epithelial cells or macrophages filled with lipid droplets that appear as “Maltese crosses” under polarized light. Fatty casts are formed when lipid-laden cells are trapped in tubular casts. Nephrotic syndrome is characterized by massive proteinuria (>3.5 g/day), hypoalbuminemia, edema, and hyperlipidemia. Common causes include minimal change disease, focal segmental glomerulosclerosis (FSGS), membranous nephropathy, and diabetic nephropathy. This patient would require quantification of proteinuria with a 24-hour urine collection or spot urine protein-to-creatinine ratio, along with further workup including kidney biopsy to determine the specific etiology.
Key Learning Point: Oval fat bodies and fatty casts on urine microscopy are pathognomonic for nephrotic syndrome and indicate glomerular disease with heavy proteinuria.
Question 3
A 55-year-old man with eGFR of 25 mL/min/1.73 m² requires imaging to evaluate a renal mass. Which imaging modality is most appropriate?
A. CT scan with intravenous iodinated contrast
B. MRI with gadolinium contrast
C. Non-contrast CT or MRI without gadolinium
D. Intravenous pyelography (IVP)
Correct Answer: C
Explanation:
In patients with eGFR <30 mL/min/1.73 m² (Stage 4-5 CKD), both iodinated contrast (used in CT) and gadolinium-based contrast agents (used in MRI) should be avoided whenever possible. Iodinated contrast carries a significant risk of contrast-induced nephropathy (CIN), which can precipitate acute kidney injury and potentially lead to dialysis dependence. Gadolinium-based contrast agents are contraindicated in severe CKD due to the risk of nephrogenic systemic fibrosis (NSF), a rare but serious condition causing fibrosis of skin, joints, and internal organs. Non-contrast CT provides excellent anatomic detail and is the gold standard for detecting kidney stones and characterizing renal masses. Non-contrast MRI can also provide good soft-tissue characterization without the risks associated with contrast agents. If contrast is absolutely necessary, the benefits must clearly outweigh the risks, and measures such as adequate hydration and using the lowest possible contrast dose should be employed.
Key Learning Point: Avoid both iodinated and gadolinium-based contrast agents in patients with eGFR <30 mL/min/1.73 m². Use non-contrast imaging modalities whenever possible.
Question 4
A 38-year-old woman presents with acute kidney injury. Urine microscopy shows red blood cell casts. What does this finding indicate?
A. Lower urinary tract bleeding (bladder or urethra)
B. Glomerular disease or glomerulonephritis
C. Acute tubular necrosis
D. Urinary tract infection
Correct Answer: B
Explanation:
Red blood cell (RBC) casts are pathognomonic for glomerular disease or glomerulonephritis. Casts are formed in the renal tubules, so their presence indicates that the pathology originates in the kidney itself rather than the lower urinary tract. RBC casts form when red blood cells pass through the glomerular basement membrane (due to glomerular inflammation or damage), enter the tubules, and become trapped in a protein matrix (Tamm-Horsfall protein) to form casts. The presence of RBC casts indicates active glomerular inflammation and requires urgent evaluation for rapidly progressive glomerulonephritis (RPGN), which can lead to irreversible kidney damage if not treated promptly. Common causes include ANCA-associated vasculitis, anti-GBM disease (Goodpasture syndrome), lupus nephritis, and post-infectious glomerulonephritis. This patient requires immediate further workup including serologic testing (ANA, ANCA, anti-GBM antibodies, complement levels) and likely kidney biopsy.
Key Learning Point: Red blood cell casts indicate glomerular disease and require urgent evaluation for rapidly progressive glomerulonephritis. They are never seen in lower urinary tract bleeding.
Question 5
A 60-year-old man with hypertension has a serum creatinine of 1.8 mg/dL and eGFR of 38 mL/min/1.73 m². A repeat measurement 4 months later shows creatinine 1.9 mg/dL and eGFR 36 mL/min/1.73 m². What stage of CKD does he have?
A. Stage 2 CKD
B. Stage 3a CKD
C. Stage 3b CKD
D. Stage 4 CKD
Correct Answer: C
Explanation:
According to the KDIGO (Kidney Disease: Improving Global Outcomes) classification, chronic kidney disease is staged based on eGFR levels. The stages are: Stage 1 (eGFR ≥90 with kidney damage), Stage 2 (eGFR 60-89 with kidney damage), Stage 3a (eGFR 45-59), Stage 3b (eGFR 30-44), Stage 4 (eGFR 15-29), and Stage 5 (eGFR <15 or on dialysis). This patient has an eGFR of 36 mL/min/1.73 m², which falls into the Stage 3b category. Importantly, the diagnosis of chronic kidney disease requires that the abnormality (reduced eGFR or evidence of kidney damage such as albuminuria) be present for at least 3 months. This patient has had two measurements 4 months apart, both showing eGFR in the 30-44 range, confirming chronicity. Stage 3b CKD indicates moderate to severe reduction in kidney function and requires nephrology referral for co-management, optimization of blood pressure control, cardiovascular risk reduction, and preparation for potential progression to Stage 4-5 CKD.
Key Learning Point: CKD staging is based on eGFR, and chronicity must be established with abnormalities present for ≥3 months. Stage 3b (eGFR 30-44) warrants nephrology referral.
Question 6
A 50-year-old woman with lupus presents with worsening kidney function. Her eGFR is 40 mL/min/1.73 m², and she has nephrotic-range proteinuria. What is the most appropriate next step to guide treatment?
A. Start empiric immunosuppression
B. Perform kidney biopsy
C. Start dialysis
D. Observe and repeat labs in 3 months
Correct Answer: B
Explanation:
Kidney biopsy is the gold standard for diagnosing the specific type and severity of lupus nephritis and is essential for guiding treatment decisions. Lupus can cause several different classes of nephritis (Class I through VI according to the International Society of Nephrology/Renal Pathology Society classification), each with different prognosis and treatment approaches. For example, Class III and IV (proliferative lupus nephritis) require aggressive immunosuppression with cyclophosphamide or mycophenolate mofetil, while Class V (membranous lupus nephritis) may be managed more conservatively. The biopsy also provides information about the extent of active inflammation versus chronic scarring (fibrosis), which helps determine prognosis and the likelihood of response to treatment. In this patient with declining kidney function and heavy proteinuria, a biopsy is indicated to determine the class of lupus nephritis, assess activity and chronicity, and guide the intensity of immunosuppressive therapy. Starting empiric treatment without histologic confirmation could lead to either under-treatment (if aggressive disease is present) or over-treatment with unnecessary immunosuppression toxicity.
Key Learning Point: Kidney biopsy is essential in lupus nephritis to determine the specific class, guide treatment intensity, and assess prognosis. Empiric treatment without biopsy is inappropriate.
Question 7
A 70-year-old man with Stage 3 CKD (eGFR 45 mL/min/1.73 m²) is found to have bilateral small kidneys (7.5 cm each) on ultrasound. What does this finding suggest?
A. Acute kidney injury
B. Chronic kidney disease with irreversible damage
C. Polycystic kidney disease
D. Normal variant for his age
Correct Answer: B
Explanation:
Small kidneys (typically defined as <9 cm in length) on ultrasound are a hallmark of chronic kidney disease with significant irreversible damage. Normal kidney size is approximately 10-12 cm in length. As CKD progresses, kidneys undergo atrophy and shrinkage due to chronic ischemia, fibrosis, and loss of nephrons. The finding of bilaterally small kidneys indicates chronicity and suggests that the kidney damage is longstanding and largely irreversible. This has important clinical implications: (1) it confirms that the patient has CKD rather than acute kidney injury, (2) it suggests that aggressive interventions to reverse kidney damage are unlikely to be beneficial, and (3) it may be a relative contraindication to kidney biopsy (as the yield is low and the risk of complications is higher in small, fibrotic kidneys). In contrast, acute kidney injury typically presents with normal or enlarged kidneys. Polycystic kidney disease causes enlarged kidneys with multiple cysts, not small kidneys. This patient’s small kidneys confirm chronic, advanced kidney disease and should prompt focus on slowing progression, managing complications, and preparing for eventual renal replacement therapy.
Key Learning Point: Bilaterally small kidneys (<9 cm) on ultrasound indicate chronic kidney disease with irreversible damage and confirm chronicity rather than acute injury.
Question 8
A 42-year-old woman has persistent microscopic hematuria and proteinuria. Urine microscopy shows dysmorphic red blood cells. What does this finding indicate?
A. Urinary tract infection
B. Kidney stones
C. Glomerular source of hematuria
D. Bladder cancer
Correct Answer: C
Explanation:
Dysmorphic red blood cells (RBCs) indicate a glomerular source of hematuria. As RBCs pass through the damaged glomerular basement membrane and travel through the nephron, they undergo morphologic changes due to osmotic and mechanical stress, resulting in irregular shapes, membrane blebs, and fragmentation. The most characteristic dysmorphic RBC is the acanthocyte (also called G1 cell), which has ring-like projections. In contrast, isomorphic (uniform, normal-appearing) RBCs suggest bleeding from the lower urinary tract (bladder, urethra, or ureters) where RBCs do not undergo the same mechanical stress. The presence of dysmorphic RBCs, especially when accompanied by proteinuria, strongly suggests glomerular disease such as IgA nephropathy, Alport syndrome, thin basement membrane disease, or other forms of glomerulonephritis. This patient requires further evaluation with serologic testing (complement levels, ANA, ANCA), assessment of kidney function, quantification of proteinuria, and possibly kidney biopsy to establish a specific diagnosis. Urinary tract infection, kidney stones, and bladder cancer typically cause isomorphic hematuria from the lower urinary tract.
Key Learning Point: Dysmorphic red blood cells indicate glomerular hematuria and suggest glomerular disease requiring further nephrology evaluation. Isomorphic RBCs suggest lower urinary tract bleeding.
Question 9
A 55-year-old man with diabetes and eGFR of 28 mL/min/1.73 m² is being managed by his primary care physician. When should he be referred to nephrology?
A. Only when he needs dialysis
B. When eGFR drops below 15 mL/min/1.73 m²
C. He should be referred now (eGFR <30)
D. Nephrology referral is not necessary for diabetic kidney disease
Correct Answer: C
Explanation:
Nephrology referral is recommended when eGFR falls below 30 mL/min/1.73 m² (Stage 4-5 CKD), and this patient with eGFR of 28 clearly meets this criterion. Early nephrology referral (at Stage 4 CKD) has been shown to improve outcomes, with studies demonstrating a 20-30% reduction in mortality compared to late referral. Benefits of early referral include: (1) timely placement of dialysis access (arteriovenous fistula creation 6-12 months before anticipated dialysis need), (2) early transplant evaluation and living donor search, (3) optimization of medical management including RAAS blockade, SGLT2 inhibitors, and management of anemia and mineral-bone disorder, (4) comprehensive patient education about treatment options (hemodialysis, peritoneal dialysis, transplantation, conservative management), and (5) advance care planning and shared decision-making. Late referral (eGFR <15 or at the time of dialysis need) is associated with higher mortality, more emergency dialysis starts with temporary catheters, increased hospitalization rates, and reduced opportunity for transplant evaluation. All patients with Stage 4-5 CKD should be co-managed by nephrology regardless of the underlying cause.
Key Learning Point: Refer patients to nephrology when eGFR <30 mL/min/1.73 m² (Stage 4-5 CKD). Early referral improves outcomes and reduces mortality by 20-30%.
Question 10
A 48-year-old man presents with acute kidney injury and nephrotic syndrome. Kidney biopsy is being considered. Which of the following is an absolute contraindication to percutaneous kidney biopsy?
A. Platelet count of 45,000/μL
B. Blood pressure of 155/95 mmHg
C. Kidney size of 9.5 cm
D. Patient anxiety about the procedure
Correct Answer: A
Explanation:
Severe thrombocytopenia (platelet count <50,000/μL) is an absolute contraindication to percutaneous kidney biopsy due to the high risk of bleeding complications. Kidney biopsy is an invasive procedure that carries inherent bleeding risk, and adequate platelet count is essential for hemostasis. Other absolute contraindications include uncontrolled bleeding disorders (coagulopathy with elevated INR or PTT) and uncooperative patients who cannot follow breath-holding instructions. The other options represent relative contraindications that may be managed: (1) Uncontrolled hypertension (BP >160/100) increases bleeding risk, but this patient’s BP of 155/95 is borderline and can be controlled with medications before the procedure; (2) A kidney size of 9.5 cm is at the lower limit of normal but not an absolute contraindication, though smaller kidneys (<9 cm) suggest end-stage disease with low diagnostic yield; (3) Patient anxiety can be managed with pre-procedure counseling and sedation. Before proceeding with kidney biopsy, this patient would require platelet transfusion to raise the platelet count above 50,000/μL, and ideally above 75,000/μL for optimal safety.
Key Learning Point: Severe thrombocytopenia (platelets <50,000/μL) is an absolute contraindication to kidney biopsy. Correct any bleeding disorders before proceeding with biopsy.
Question 11
A 35-year-old woman presents with cola-colored urine, periorbital edema, and hypertension. Urinalysis shows 3+ blood and 2+ protein. Urine microscopy reveals red blood cell casts. What is the most likely diagnosis?
A. Urinary tract infection
B. Acute glomerulonephritis
C. Acute tubular necrosis
D. Nephrotic syndrome
Correct Answer: B
Explanation:
This patient presents with the classic triad of acute glomerulonephritis: hematuria (cola-colored urine), edema (periorbital), and hypertension. The presence of red blood cell casts on urine microscopy is pathognomonic for glomerular disease and confirms the diagnosis. Acute glomerulonephritis is characterized by acute inflammation of the glomeruli, leading to impaired filtration, fluid retention (causing edema and hypertension), and passage of red blood cells through the damaged glomerular basement membrane (causing hematuria and RBC casts). The nephritic syndrome presentation (hematuria, hypertension, edema, mild to moderate proteinuria) differs from nephrotic syndrome (massive proteinuria, severe edema, hypoalbuminemia, hyperlipidemia). Common causes of acute glomerulonephritis include post-infectious glomerulonephritis (especially post-streptococcal), IgA nephropathy, ANCA-associated vasculitis, anti-GBM disease, and lupus nephritis. This patient requires urgent evaluation with serologic testing (ASO titer, complement levels, ANA, ANCA, anti-GBM antibodies), assessment of kidney function, and possibly kidney biopsy to determine the specific etiology and guide treatment.
Key Learning Point: The combination of hematuria, edema, hypertension, and red blood cell casts indicates acute glomerulonephritis requiring urgent evaluation for rapidly progressive disease.
Question 12
A 62-year-old man with chronic kidney disease has the following lab results: eGFR 32 mL/min/1.73 m², hemoglobin 9.5 g/dL, calcium 8.2 mg/dL, phosphorus 5.8 mg/dL, PTH 180 pg/mL. What complication of CKD do these findings represent?
A. Acute kidney injury
B. CKD-mineral and bone disorder (CKD-MBD)
C. Nephrotic syndrome
D. Uremic syndrome
Correct Answer: B
Explanation:
This patient demonstrates classic findings of CKD-mineral and bone disorder (CKD-MBD), a common complication of advanced chronic kidney disease. The constellation of findings includes: (1) hyperphosphatemia (phosphorus 5.8 mg/dL, normal 2.5-4.5 mg/dL), (2) hypocalcemia (calcium 8.2 mg/dL, normal 8.5-10.5 mg/dL), and (3) secondary hyperparathyroidism (PTH 180 pg/mL, elevated). The pathophysiology involves: As kidney function declines, phosphorus excretion decreases, leading to hyperphosphatemia. Elevated phosphorus binds calcium, causing hypocalcemia. The kidneys also fail to produce adequate 1,25-dihydroxyvitamin D (active vitamin D), further reducing calcium absorption from the gut. In response to hypocalcemia and hyperphosphatemia, the parathyroid glands increase PTH secretion (secondary hyperparathyroidism) in an attempt to maintain calcium homeostasis. Chronic elevation of PTH leads to renal osteodystrophy (bone disease), vascular calcification, and increased cardiovascular risk. Management includes dietary phosphorus restriction, phosphate binders, vitamin D supplementation, and calcimimetics (such as cinacalcet) to control PTH. The anemia (hemoglobin 9.5 g/dL) is also a complication of CKD due to reduced erythropoietin production.
Key Learning Point: CKD-mineral and bone disorder is characterized by hyperphosphatemia, hypocalcemia, and secondary hyperparathyroidism. It requires active management to prevent bone disease and cardiovascular complications.
Question 13
A 40-year-old woman with systemic lupus erythematosus develops worsening proteinuria. Urine protein-to-creatinine ratio is 4.5 g/g. What is the most appropriate method to quantify her proteinuria?
A. Urine dipstick alone
B. Spot urine protein-to-creatinine ratio (already done)
C. 24-hour urine collection
D. Serum albumin measurement
Correct Answer: B
Explanation:
The spot urine protein-to-creatinine ratio (UPCR) is the most appropriate and convenient method for quantifying proteinuria in clinical practice. This patient has already had a UPCR performed, which shows 4.5 g/g, indicating nephrotic-range proteinuria (>3.5 g/day). The UPCR correlates well with 24-hour urine protein excretion, with the ratio approximately equal to grams of protein excreted per day. For example, a UPCR of 4.5 g/g indicates approximately 4.5 grams of protein loss per day. The advantages of UPCR over 24-hour urine collection include: (1) convenience (single spot urine sample rather than collecting all urine for 24 hours), (2) improved patient compliance, (3) rapid results, and (4) avoidance of collection errors (incomplete collection, timing errors). While 24-hour urine collection remains the gold standard for precise quantification, UPCR is now the preferred method in most clinical situations and is recommended by KDIGO guidelines. Urine dipstick is only semi-quantitative and cannot accurately measure the degree of proteinuria. Serum albumin reflects the consequence of proteinuria (hypoalbuminemia) but does not quantify urinary protein loss.
Key Learning Point: Spot urine protein-to-creatinine ratio (UPCR) is the preferred method for quantifying proteinuria. A UPCR >3.5 g/g indicates nephrotic-range proteinuria.
Question 14
A 58-year-old man with polycystic kidney disease has an eGFR of 35 mL/min/1.73 m². Renal ultrasound shows bilaterally enlarged kidneys with multiple cysts. Which imaging modality would be most appropriate for further evaluation of a suspected renal mass?
A. CT with contrast
B. MRI with gadolinium
C. MRI without gadolinium
D. Intravenous pyelography
Correct Answer: C
Explanation:
In this patient with eGFR of 35 mL/min/1.73 m² (Stage 3b CKD) and polycystic kidney disease, MRI without gadolinium is the most appropriate imaging modality. Patients with polycystic kidney disease have an increased risk of renal cell carcinoma, and distinguishing between benign cysts and solid masses can be challenging. MRI provides superior soft-tissue contrast compared to ultrasound and can better characterize renal masses. Importantly, in patients with eGFR <30-45 mL/min/1.73 m², both iodinated contrast (CT) and gadolinium-based contrast agents (MRI) should be avoided when possible. Iodinated contrast carries risk of contrast-induced nephropathy, while gadolinium is contraindicated in severe CKD (eGFR <30) due to the risk of nephrogenic systemic fibrosis (NSF). Although this patient’s eGFR of 35 is borderline, caution is warranted, and non-contrast MRI is the safest option. Non-contrast MRI can utilize techniques such as T2-weighted imaging and diffusion-weighted imaging to characterize masses without the need for contrast. If contrast is absolutely necessary, the risks and benefits must be carefully weighed, and the lowest possible dose should be used with adequate hydration.
Key Learning Point: In patients with reduced kidney function (eGFR <45), MRI without gadolinium is preferred for characterizing renal masses. Avoid both iodinated and gadolinium contrast when possible.
Question 15
A 52-year-old man with hypertension presents with serum creatinine of 2.8 mg/dL (247 μmol/L). He has no prior creatinine measurements. Renal ultrasound shows normal-sized kidneys (11 cm bilaterally) with increased echogenicity. What is the most appropriate next step?
A. Diagnose chronic kidney disease and refer to nephrology
B. Repeat creatinine in 3 months to establish chronicity
C. Perform urgent kidney biopsy
D. Start dialysis immediately
Correct Answer: B
Explanation:
While this patient has significantly elevated creatinine (2.8 mg/dL, likely corresponding to eGFR 20-30 mL/min/1.73 m²), the diagnosis of chronic kidney disease requires that kidney dysfunction be present for at least 3 months. Since this is his first creatinine measurement and there is no prior baseline, it is impossible to determine whether this represents acute kidney injury, chronic kidney disease, or acute-on-chronic kidney disease. The ultrasound findings provide some clues: normal-sized kidneys suggest that the process may not be longstanding (as chronic kidney disease typically causes kidney atrophy and shrinkage), while increased echogenicity can be seen in both acute and chronic kidney disease. The most appropriate next step is to repeat the creatinine measurement in 3 months (or sooner if clinical concern for acute kidney injury exists) to establish whether the dysfunction is chronic. During this period, the patient should undergo further evaluation including urinalysis, urine microscopy, assessment for proteinuria, and investigation for reversible causes of kidney dysfunction. If repeat testing in 3 months shows persistent elevation, the diagnosis of CKD would be confirmed, and nephrology referral would be appropriate. Immediate dialysis is not indicated unless the patient has life-threatening complications of kidney failure (severe hyperkalemia, pulmonary edema, uremic pericarditis).
Key Learning Point: The diagnosis of chronic kidney disease requires abnormalities present for ≥3 months. Repeat testing is necessary to establish chronicity when prior baseline is unknown.
Question 16
A 44-year-old woman with nephrotic syndrome undergoes kidney biopsy. The pathology report describes “diffuse foot process effacement on electron microscopy with no immune complex deposits.” What is the most likely diagnosis?
A. Membranous nephropathy
B. Minimal change disease
C. Focal segmental glomerulosclerosis (FSGS)
D. IgA nephropathy
Correct Answer: B
Explanation:
The pathology findings of diffuse foot process effacement (also called podocyte effacement) on electron microscopy with absence of immune complex deposits are characteristic of minimal change disease (MCD). Minimal change disease is the most common cause of nephrotic syndrome in children and also occurs in adults. The hallmark pathologic feature is effacement (flattening) of the podocyte foot processes seen on electron microscopy, while light microscopy appears essentially normal (hence the name “minimal change”). Immunofluorescence is negative for immune complex deposits, distinguishing it from other causes of nephrotic syndrome. MCD typically presents with sudden onset of nephrotic syndrome (heavy proteinuria, edema, hypoalbuminemia, hyperlipidemia) and is highly responsive to corticosteroid therapy, with most patients achieving complete remission. In contrast, membranous nephropathy shows subepithelial immune complex deposits on electron microscopy and granular IgG and C3 on immunofluorescence. FSGS shows segmental sclerosis on light microscopy and may have focal foot process effacement. IgA nephropathy shows mesangial IgA deposits on immunofluorescence and typically presents with nephritic syndrome (hematuria) rather than nephrotic syndrome.
Key Learning Point: Minimal change disease is characterized by diffuse foot process effacement on electron microscopy with negative immunofluorescence. It is highly steroid-responsive.
Question 17
A 67-year-old man with Stage 4 CKD (eGFR 22 mL/min/1.73 m²) requires evaluation for suspected renovascular hypertension. Which imaging study is most appropriate?
A. CT angiography with iodinated contrast
B. MR angiography with gadolinium
C. Captopril renography (nuclear medicine)
D. Renal arteriography with iodinated contrast
Correct Answer: C
Explanation:
In patients with advanced chronic kidney disease (Stage 4-5, eGFR <30 mL/min/1.73 m²), captopril renography (nuclear medicine study) is the most appropriate imaging modality for evaluating renovascular hypertension (renal artery stenosis). This functional imaging study uses radiopharmaceuticals (typically Tc-99m MAG3) to assess renal perfusion and function before and after administration of captopril (an ACE inhibitor). In patients with hemodynamically significant renal artery stenosis, captopril causes a marked decrease in GFR in the affected kidney, which is detected on the scan. The key advantage of captopril renography is that it does not require nephrotoxic contrast agents, making it safe in patients with reduced kidney function. In contrast, CT angiography requires iodinated contrast (risk of contrast-induced nephropathy), MR angiography typically requires gadolinium (risk of nephrogenic systemic fibrosis in eGFR <30), and conventional renal arteriography is invasive and uses iodinated contrast. Alternative non-contrast options include Doppler ultrasound (operator-dependent and limited by body habitus) and non-contrast MR angiography (available at some centers but less widely accessible). If revascularization is being considered, renal arteriography may ultimately be necessary, but diagnostic evaluation should start with non-invasive, contrast-free methods.
Key Learning Point: In patients with advanced CKD (eGFR <30), use captopril renography or Doppler ultrasound to evaluate for renovascular hypertension. Avoid contrast-based imaging when possible.
Question 18
A 30-year-old man presents with gross hematuria following an upper respiratory infection. Urinalysis shows 3+ blood and 1+ protein. Urine microscopy reveals dysmorphic RBCs and RBC casts. Serum IgA level is elevated. What is the most likely diagnosis?
A. Post-streptococcal glomerulonephritis
B. IgA nephropathy (Berger disease)
C. Alport syndrome
D. Thin basement membrane disease
Correct Answer: B
Explanation:
The clinical presentation of gross hematuria occurring concurrently with or immediately following an upper respiratory infection (synpharyngitic hematuria) in a young adult, combined with dysmorphic RBCs, RBC casts, and elevated serum IgA, is classic for IgA nephropathy (also called Berger disease). IgA nephropathy is the most common primary glomerulonephritis worldwide and typically presents with episodic gross hematuria triggered by mucosal infections (upper respiratory or gastrointestinal). The pathophysiology involves deposition of IgA immune complexes in the glomerular mesangium, leading to glomerular inflammation. Kidney biopsy shows mesangial IgA deposits on immunofluorescence and mesangial proliferation on light microscopy. The key distinguishing feature from post-streptococcal glomerulonephritis is the timing: IgA nephropathy causes hematuria during or immediately after infection (synpharyngitic), while post-streptococcal GN has a latent period of 1-3 weeks after streptococcal infection. Alport syndrome is a hereditary nephritis with progressive kidney disease, hearing loss, and eye abnormalities. Thin basement membrane disease causes persistent microscopic hematuria but rarely gross hematuria or significant proteinuria. Most patients with IgA nephropathy have a benign course, but some develop progressive CKD requiring treatment with RAAS blockade and immunosuppression.
Key Learning Point: IgA nephropathy presents with synpharyngitic hematuria (concurrent with upper respiratory infection) and is the most common primary glomerulonephritis worldwide.
Question 19
A 56-year-old woman with diabetes and CKD Stage 3b (eGFR 38 mL/min/1.73 m²) is started on an SGLT2 inhibitor. Two weeks later, her creatinine increases from 1.6 to 1.9 mg/dL and eGFR drops to 32 mL/min/1.73 m². What is the most appropriate management?
A. Discontinue the SGLT2 inhibitor immediately
B. Continue the SGLT2 inhibitor and recheck in 4 weeks
C. Increase the dose of the SGLT2 inhibitor
D. Start dialysis
Correct Answer: B
Explanation:
A mild, transient increase in creatinine (typically 10-20%) after starting an SGLT2 inhibitor is expected and does not indicate kidney injury. This phenomenon is called “hemodynamic creatinine increase” and reflects the drug’s mechanism of action. SGLT2 inhibitors block glucose reabsorption in the proximal tubule, leading to increased sodium delivery to the macula densa, which triggers tubuloglomerular feedback and causes afferent arteriolar vasoconstriction. This reduces intraglomerular pressure and hyperfiltration, which is beneficial for long-term kidney protection but causes a temporary decrease in GFR and rise in creatinine. This initial creatinine increase is not harmful and actually predicts better long-term kidney outcomes. Clinical trials of SGLT2 inhibitors (CREDENCE, DAPA-CKD, EMPA-KIDNEY) have consistently shown that patients who experience this initial creatinine rise have greater long-term kidney protection and slower CKD progression. The SGLT2 inhibitor should be continued, and kidney function should be rechecked in 4 weeks. If creatinine continues to rise significantly (>30% increase) or if there are other signs of acute kidney injury, further evaluation would be warranted. SGLT2 inhibitors are now a cornerstone of CKD management in patients with diabetes and have been shown to reduce CKD progression, cardiovascular events, and mortality.
Key Learning Point: A mild transient creatinine increase (10-20%) after starting SGLT2 inhibitors is expected and beneficial. Continue the medication and recheck in 4 weeks.
Question 20
A 72-year-old man with CKD Stage 4 (eGFR 24 mL/min/1.73 m²) presents with severe fatigue, nausea, pruritus, and confusion. Laboratory studies show BUN 95 mg/dL, creatinine 6.2 mg/dL, potassium 5.8 mEq/L, bicarbonate 14 mEq/L. What is the most appropriate next step?
A. Continue conservative management and recheck labs in 1 month
B. Urgent nephrology referral for dialysis evaluation
C. Start oral phosphate binders
D. Increase diuretic dose
Correct Answer: B
Explanation:
This patient presents with uremic syndrome, a clinical constellation of symptoms resulting from the accumulation of uremic toxins in advanced kidney failure. The symptoms include fatigue, nausea, vomiting, anorexia, pruritus (itching), altered mental status (confusion, lethargy), pericarditis, and bleeding tendency. Laboratory findings show severe kidney dysfunction (eGFR 24, creatinine 6.2, BUN 95), hyperkalemia (potassium 5.8), and metabolic acidosis (bicarbonate 14). Uremic syndrome is an indication for urgent dialysis initiation, as it represents life-threatening kidney failure that cannot be managed conservatively. According to guidelines, dialysis should be initiated when patients develop uremic symptoms, regardless of the absolute GFR level. Other absolute indications for urgent dialysis include severe hyperkalemia (>6.5 mEq/L or with ECG changes), severe metabolic acidosis (pH <7.1), pulmonary edema refractory to diuretics, and uremic pericarditis. This patient requires urgent nephrology referral for dialysis initiation, likely starting with hemodialysis via temporary catheter given the urgent nature. Conservative management is inappropriate when uremic symptoms are present. While phosphate binders and diuretics may be part of CKD management, they do not address the immediate life-threatening complications of uremia.
Key Learning Point: Uremic syndrome (nausea, confusion, pruritus, fatigue in advanced CKD) is an indication for urgent dialysis initiation regardless of absolute GFR level.
Answer Key Summary
1.B – Calculate eGFR
2.B – Nephrotic syndrome
3.C – Non-contrast CT or MRI without gadolinium
4.B – Glomerular disease or glomerulonephritis
5.C – Stage 3b CKD
6.B – Perform kidney biopsy
7.B – Chronic kidney disease with irreversible damage
8.C – Glomerular source of hematuria
9.C – He should be referred now (eGFR <30)
10.A – Platelet count of 45,000/μL
11.B – Acute glomerulonephritis
12.B – CKD-mineral and bone disorder (CKD-MBD)
13.B – Spot urine protein-to-creatinine ratio
14.C – MRI without gadolinium
15.B – Repeat creatinine in 3 months to establish chronicity
16.B – Minimal change disease
17.C – Captopril renography (nuclear medicine)
18.B – IgA nephropathy (Berger disease)
19.B – Continue the SGLT2 inhibitor and recheck in 4 weeks
20.B – Urgent nephrology referral for dialysis evaluation