Introduction
Fluid and electrolyte homeostasis is a cornerstone of human physiology, and its derangement is a common feature in a wide array of clinical conditions, particularly in the realm of nephrology. The kidneys play a central role in maintaining the delicate balance of fluids, electrolytes, and acid-base status. Therefore, any dysfunction in the renal system can lead to significant and often life-threatening imbalances. This chapter will provide a comprehensive overview of the most common fluid and electrolyte disorders encountered in clinical practice, with a focus on their pathophysiology, clinical presentation, diagnosis, and management, tailored for the practicing nephrologist.
Sodium Disorders
Sodium is the most abundant cation in the extracellular fluid and a primary determinant of plasma osmolality. Disorders of sodium balance are, therefore, disorders of water balance. The regulation of sodium and water is intricately linked through the thirst mechanism and the action of antidiuretic hormone (ADH).
Hyponatremia
Hyponatremia, defined as a serum sodium concentration of less than 135 mEq/L, is the most common electrolyte abnormality observed in hospitalized patients. It is associated with significant morbidity and mortality, and its management requires a careful and systematic approach.
Etiology and Classification
Hyponatremia is classified based on the patient’s volume status into hypovolemic, euvolemic, and hypervolemic hyponatremia. This classification is crucial for determining the underlying cause and guiding appropriate therapy.
| Volume Status | Urine Sodium | Causes | Clinical Features |
| Hypovolemic | < 20 mEq/L | Dehydration, diarrhea, vomiting | Orthostatic hypotension, tachycardia, dry mucous membranes |
| > 20 mEq/L | Diuretic use, salt-wasting nephropathy | Signs of volume depletion | |
| Euvolemic | > 20 mEq/L | SIADH, hypothyroidism, adrenal insufficiency | No signs of volume overload or depletion |
| Hypervolemic | < 20 mEq/L | Heart failure, cirrhosis, nephrotic syndrome | Edema, ascites, pulmonary congestion |
| > 20 mEq/L | Acute or chronic renal failure | Signs of volume overload |
Clinical Features
The clinical manifestations of hyponatremia are primarily neurological and depend on the severity and acuity of the sodium reduction. Symptoms can range from mild headache, nausea, and confusion to severe manifestations such as seizures, coma, and respiratory arrest.
Diagnostic Approach
The diagnostic workup of hyponatremia involves a thorough history and physical examination, along with laboratory tests including serum and urine osmolality, and urine sodium concentration. An algorithm for the diagnostic approach is presented below:
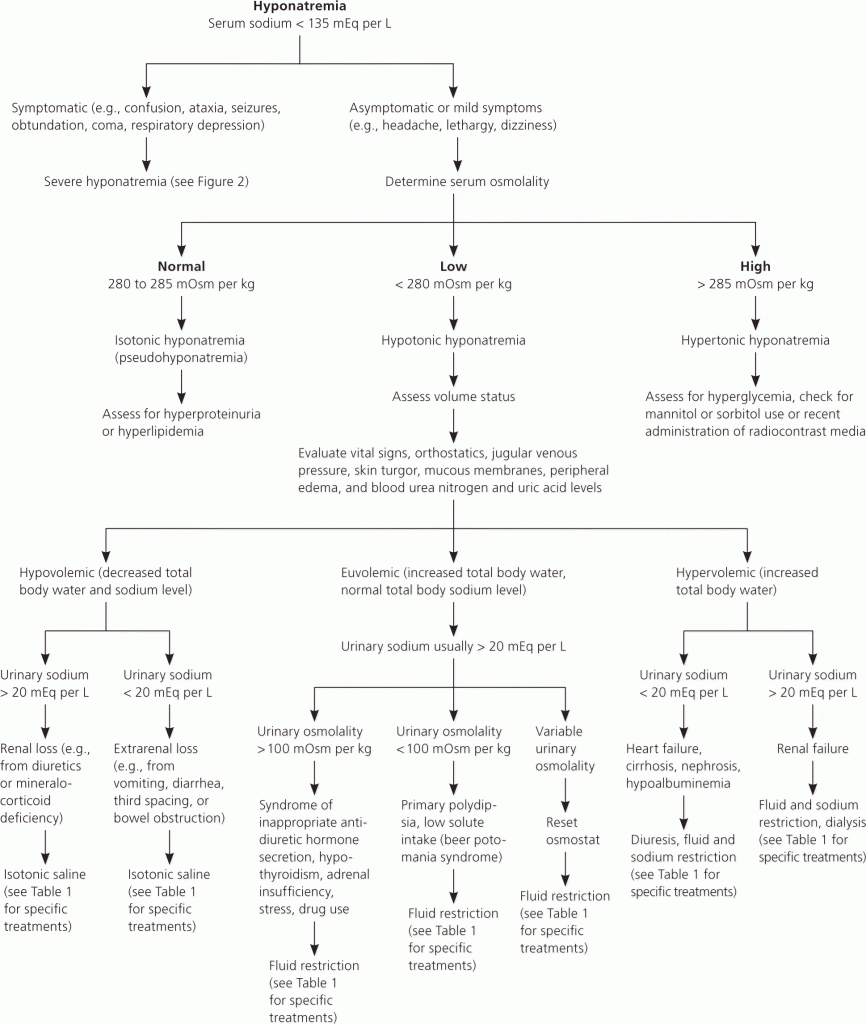
Management
The treatment of hyponatremia is dictated by the severity of symptoms, the acuity of the condition, and the underlying etiology. The primary goal is to raise the serum sodium concentration at a safe rate to prevent neurological complications, particularly osmotic demyelination syndrome (ODS).
The rate of sodium correction should be 6 to 12 mEq per L in the first 24 hours and 18 mEq per L or less in 48 hours. [14]
In cases of severe symptomatic hyponatremia, a bolus of 100-150 mL of 3% hypertonic saline can be administered. For less severe cases, fluid restriction and treatment of the underlying cause are the mainstays of therapy.
Hypernatremia
Hypernatremia, defined as a serum sodium concentration greater than 145 mEq/L, is a disorder of water deficit. It is less common than hyponatremia but carries a high mortality rate.
Etiology
Hypernatremia is most commonly seen in patients with an impaired thirst mechanism or those who are unable to access water, such as infants, elderly patients with altered mental status, and intubated patients.
Clinical Features
Symptoms of hypernatremia are also primarily neurological and include lethargy, weakness, irritability, and in severe cases, seizures and coma.
Management
The management of hypernatremia involves correcting the water deficit. The rate of correction is crucial to avoid cerebral edema.
Chronic hypernatremia should be corrected at a rate of 0.5 mEq per L per hour, with a maximum change of 8 to 10 mEq per L in a 24-hour period. [33]
The route of water replacement (oral or intravenous) depends on the patient’s clinical status.
Potassium Disorders
Potassium is the most abundant intracellular cation and is crucial for maintaining the resting membrane potential of cells, particularly cardiac and neuromuscular cells. Therefore, disorders of potassium balance can have severe consequences.
Hypokalemia
Hypokalemia, defined as a serum potassium concentration of less than 3.6 mEq/L, is a common clinical problem. It can result from decreased intake, increased excretion (renal or gastrointestinal), or a shift of potassium from the extracellular to the intracellular space.
Clinical Features
Symptoms of hypokalemia include muscle weakness, fatigue, constipation, and in severe cases, paralysis and life-threatening cardiac arrhythmias.
Management
The management of hypokalemia involves repleting potassium stores and addressing the underlying cause. The urgency and route of potassium replacement depend on the severity of hypokalemia and the presence of symptoms.
| Severity | Serum K+ (mEq/L) | Management |
| Mild | 3.0 – 3.5 | Oral potassium chloride supplementation |
| Moderate | 2.5 – 3.0 | Oral or intravenous potassium chloride |
| Severe | < 2.5 | Intravenous potassium chloride with cardiac monitoring |
For severe, symptomatic hypokalemia, intravenous potassium chloride (5-10 mEq over 15-30 minutes) is warranted. For less severe cases, oral potassium chloride is preferred.
Hyperkalemia
Hyperkalemia, defined as a serum potassium concentration greater than 5.5 mEq/L, is a medical emergency due to its potential to cause life-threatening cardiac arrhythmias.
Etiology
Hyperkalemia can be caused by increased potassium intake, decreased potassium excretion (e.g., in chronic kidney disease or with certain medications), or a shift of potassium from the intracellular to the extracellular space.
Management
The management of hyperkalemia is a stepwise approach focused on antagonizing the membrane effects of potassium, shifting potassium into cells, and removing potassium from the body.
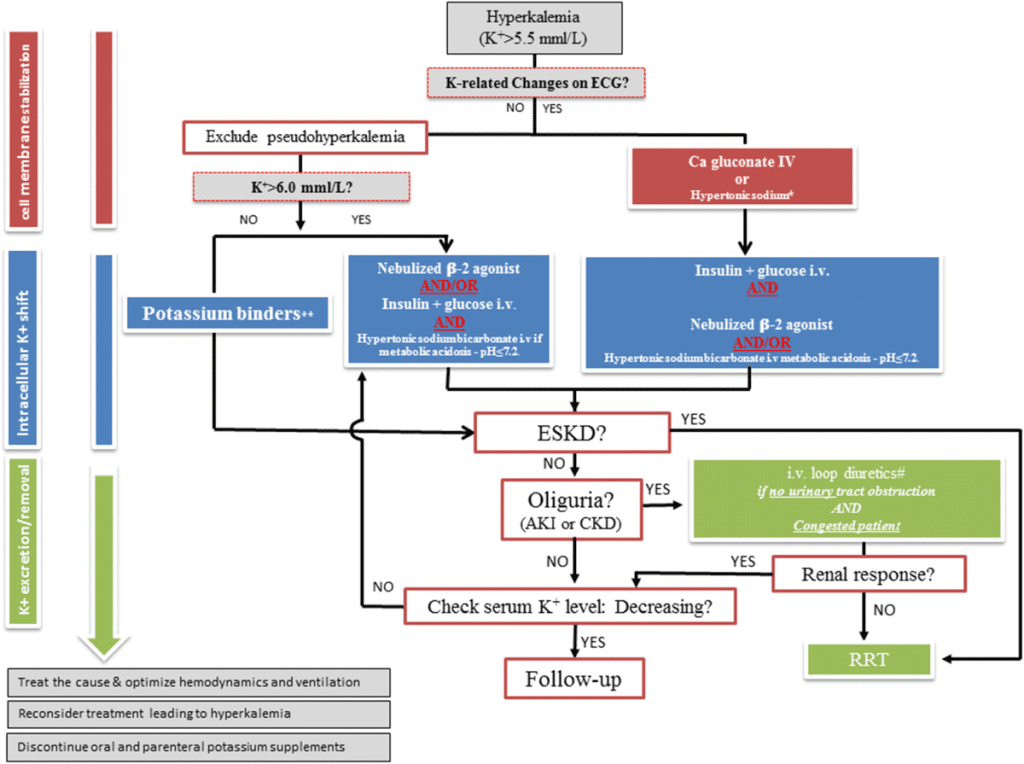
1. Stabilize the Cardiac Membrane: Intravenous calcium gluconate or calcium chloride is administered to antagonize the cardiotoxic effects of hyperkalemia.
2. Shift Potassium Intracellularly: Insulin (with glucose to prevent hypoglycemia) and beta-2 agonists (e.g., albuterol) are used to drive potassium into the cells.
3. Remove Potassium from the Body: Loop diuretics, potassium-binding resins (e.g., patiromer, sodium zirconium cyclosilicate), and dialysis are effective methods for removing potassium from the body.
Calcium, Phosphate, and Magnesium Disorders
Disorders of calcium, phosphate, and magnesium are closely intertwined and are particularly prevalent in patients with chronic kidney disease (CKD). These minerals are essential for bone health, and their dysregulation contributes to the syndrome of CKD-Mineral and Bone Disorder (CKD-MBD).
Calcium Disorders
Hypocalcemia
Hypocalcemia, a corrected serum calcium level below 8.8 mg/dL, can result from various conditions, including vitamin D deficiency, hypoparathyroidism, and CKD. It is crucial to correct the serum calcium for the albumin level, as a significant portion of calcium is protein-bound.
Hypercalcemia
Hypercalcemia, a corrected serum calcium level above 10.7 mg/dL, is most commonly caused by primary hyperparathyroidism or malignancy. In the context of nephrology, it can be seen in patients with tertiary hyperparathyroidism or adynamic bone disease.
Phosphate Disorders
Hypophosphatemia
Hypophosphatemia, a serum phosphate level below 2.5 mg/dL, can be caused by decreased intestinal absorption, increased renal excretion, or a shift of phosphate into cells. It is commonly seen in refeeding syndrome and in patients on continuous renal replacement therapy.
Hyperphosphatemia
Hyperphosphatemia, a serum phosphate level above 4.5 mg/dL, is a hallmark of advanced CKD due to impaired renal excretion. It is a major contributor to the pathogenesis of CKD-MBD and is associated with increased cardiovascular mortality.
Magnesium Disorders
Hypomagnesemia
Hypomagnesemia, a serum magnesium level below 1.46 mg/dL, can be caused by gastrointestinal or renal losses. It is often associated with other electrolyte abnormalities, such as hypokalemia and hypocalcemia, and can predispose to cardiac arrhythmias.
Hypermagnesemia
Hypermagnesemia, a serum magnesium level above 2.68 mg/dL, is most commonly seen in patients with advanced CKD and excessive magnesium intake (e.g., from antacids or laxatives). Symptoms are primarily neurological and neuromuscular, including weakness, confusion, and respiratory depression.
Acid-Base Disorders
Acid-base homeostasis is tightly regulated to maintain the blood pH within a narrow range (7.35-7.45). The kidneys and the respiratory system are the primary organs involved in this regulation. Acid-base disorders are classified as either metabolic or respiratory, and as either acidosis or alkalosis.
Metabolic Acidosis
Metabolic acidosis is characterized by a primary decrease in serum bicarbonate concentration. It is a common complication of advanced CKD. The anion gap is a useful tool for the differential diagnosis of metabolic acidosis.
Anion Gap = Sodium – (Chloride + Bicarbonate)
A high anion gap metabolic acidosis is typically caused by the accumulation of unmeasured anions, such as in diabetic ketoacidosis, lactic acidosis, and uremia. A normal anion gap metabolic acidosis is caused by the loss of bicarbonate, as seen in diarrhea or renal tubular acidosis.
Metabolic Alkalosis
Metabolic alkalosis is characterized by a primary increase in serum bicarbonate concentration. It can be caused by loss of hydrogen ions (e.g., vomiting) or administration of alkali.
Respiratory Acidosis and Alkalosis
Respiratory acid-base disorders are caused by alterations in the partial pressure of carbon dioxide (pCO2). Respiratory acidosis results from hypoventilation and an increase in pCO2, while respiratory alkalosis results from hyperventilation and a decrease in pCO2.
Summary
Fluid and electrolyte disorders are a frequent and challenging aspect of nephrology practice. A thorough understanding of the underlying pathophysiology and a systematic approach to diagnosis and management are essential for optimal patient outcomes. This chapter has provided a comprehensive overview of the most common disorders of sodium, potassium, calcium, phosphate, magnesium, and acid-base balance.
Clinical Pearls
•Hyponatremia is a disorder of water balance, not sodium balance.
•The rate of correction of sodium disorders is critical to prevent neurological complications.
•Hyperkalemia is a medical emergency that requires immediate intervention.
•In patients with CKD, disorders of calcium, phosphate, and magnesium are common and contribute to CKD-MBD.
•The anion gap is a valuable tool in the differential diagnosis of metabolic acidosis.
References
1.Shrimanker, I., & Bhattarai, S. (2023). Electrolytes. In StatPearls. StatPearls Publishing. https://www.ncbi.nlm.nih.gov/books/NBK541123/
2.Deabes, A. A. (2024). Fluid and electrolyte imbalance in renal dysfunction. Journal of Renal and Hepatic Disorders, 8(1), 1-10. https://www.sciencedirect.com/science/article/pii/S1472029924000432
3.Braun, M. M., Barstow, C. H., & Pyzocha, N. J. (2015). Diagnosis and management of sodium disorders: hyponatremia and hypernatremia. American Family Physician, 91(5), 299-307. https://www.aafp.org/pubs/afp/issues/2015/0301/p299.html
4.Kim, M. J., Valerio, C., & Knobloch, G. K. (2023). Potassium disorders: hypokalemia and hyperkalemia. American Family Physician, 107(1), 59-70. https://www.aafp.org/pubs/afp/issues/2023/0100/potassium-disorders-hypokalemia-hyperkalemia.html
5.Simon, L. V., Hashmi, M. F., & Farrell, M. W. (2023). Hyperkalemia. In StatPearls. StatPearls Publishing. https://www.ccjm.org/content/86/1/69
6.Murray, S. L., & Tonthat, S. (2024). Calcium and Phosphate Disorders: Core Curriculum 2024. American Journal of Kidney Diseases, 83(2), 257-272. https://www.ajkd.org/article/S0272-6386(23)00772-2/fulltext
7.Medbullets Step 1. (n.d.). Acid-Base Nomogram. https://step1.medbullets.com/renal/105026/acid-base-nomogram
Detailed Management Protocols
Hyponatremia Management Protocol
Acute Symptomatic Hyponatremia (< 48 hours)
Clinical Presentation:
•Severe neurological symptoms: seizures, altered mental status, coma
•Serum sodium typically < 125 mEq/L
•Requires immediate intervention
Emergency Management:
1.Initial Assessment
•Confirm diagnosis with repeat serum sodium
•Assess volume status (hypovolemic, euvolemic, hypervolemic)
•Obtain urine sodium, urine osmolality, serum osmolality
•Rule out pseudohyponatremia (hyperglycemia, hyperlipidemia, hyperproteinemia)
2.3% Hypertonic Saline Protocol
•Bolus approach: 100-150 mL IV push over 10-20 minutes
•Repeat: Can be repeated 2-3 times if symptoms persist
•Target: Increase serum Na+ by 4-6 mEq/L in first 1-2 hours
•Maximum correction: 6-12 mEq/L in first 24 hours, ≤18 mEq/L in 48 hours
3.Calculation of Sodium Deficit
4.Monitoring
•Check serum sodium every 2-4 hours initially
•Continuous cardiac monitoring if severe
•Neurological assessments every 1-2 hours
•Strict intake/output monitoring
5.Prevention of Overcorrection
•If correction exceeds 6 mEq/L in 6 hours or 12 mEq/L in 24 hours:
•Stop hypertonic saline immediately
•Consider desmopressin (DDAVP) 2-4 mcg IV/SC
•Administer hypotonic fluids (D5W)
•Monitor sodium every 2 hours
Chronic Hyponatremia (> 48 hours)
Management Strategy:
•More conservative approach due to brain adaptation
•Target correction: 4-6 mEq/L per 24 hours
•Maximum: 8 mEq/L in 24 hours, 18 mEq/L in 48 hours
SIADH Diagnostic Criteria:
1.Serum osmolality < 275 mOsm/kg
2.Urine osmolality > 100 mOsm/kg (inappropriately concentrated)
3.Urine sodium > 40 mEq/L (with normal salt intake)
4.Clinical euvolemia
5.Normal thyroid and adrenal function
6.No recent diuretic use
SIADH Treatment Algorithm:
| Severity | Serum Na+ | Treatment |
| Mild | 130-135 mEq/L | Fluid restriction (800-1000 mL/day) |
| Moderate | 125-130 mEq/L | Fluid restriction + salt tablets (1-3 g/day) |
| Severe | < 125 mEq/L | Consider vaptans (tolvaptan 15 mg daily) |
| Refractory | Any | Urea 15-30 g/day or demeclocycline 600-1200 mg/day |
Vaptan Therapy:
•Tolvaptan: Start 15 mg daily, max 60 mg/day
•Contraindications: Hypovolemic hyponatremia, anuria, urgent need for rapid correction
•Monitoring: Check sodium 6-8 hours after first dose, then every 6-8 hours for 24 hours
•Risk: Overly rapid correction – requires hospitalization for initiation
Hyperkalemia Management Protocol
ECG Changes in Hyperkalemia (Progressive)
| K+ Level | ECG Changes |
| 5.5-6.5 mEq/L | Tall, peaked T waves (narrow base) |
| 6.5-7.5 mEq/L | Prolonged PR interval, flattened P waves |
| 7.5-8.0 mEq/L | Widened QRS complex, loss of P waves |
| > 8.0 mEq/L | Sine wave pattern, ventricular fibrillation, asystole |
Emergency Management Protocol
Step 1: Cardiac Membrane Stabilization (if ECG changes present)
•Calcium gluconate 10%: 1-2 grams (10-20 mL) IV over 2-5 minutes
•Calcium chloride 10%: 0.5-1 gram (5-10 mL) IV over 2-5 minutes (via central line preferred)
•Onset: 1-3 minutes
•Duration: 30-60 minutes
•Repeat: If ECG changes persist after 5 minutes
•Note: Does NOT lower potassium, only protects the heart
Step 2: Shift Potassium Intracellularly
| Agent | Dose | Onset | Duration | Expected K+ Decrease |
| Regular Insulin + Glucose | 10 units IV + 25g D50W (50 mL) | 15-30 min | 4-6 hours | 0.5-1.2 mEq/L |
| Albuterol nebulized | 10-20 mg (4-8 puffs) | 30-90 min | 2-4 hours | 0.5-1.0 mEq/L |
| Sodium bicarbonate | 50-100 mEq IV (if acidotic) | 30-60 min | 2-4 hours | 0.5-1.0 mEq/L |
Important Notes:
•Monitor glucose every 30-60 minutes after insulin (risk of hypoglycemia)
•Albuterol less effective in ESRD patients
•Bicarbonate only effective if metabolic acidosis present (pH < 7.2)
•Can use insulin + albuterol together for additive effect
Step 3: Remove Potassium from Body
| Method | Dose/Protocol | Onset | Efficacy |
| Loop diuretics | Furosemide 40-80 mg IV | 30-60 min | Effective if GFR > 30 |
| Sodium zirconium cyclosilicate (Lokelma) | 10 g PO TID × 48h, then 10 g daily | 1-2 hours | 0.7-1.0 mEq/L decrease |
| Patiromer (Veltassa) | 8.4 g PO daily (max 25.2 g) | 4-7 hours | 0.5-1.0 mEq/L decrease |
| Sodium polystyrene sulfonate (Kayexalate) | 15-30 g PO/PR | 2-4 hours | Variable, GI risks |
| Hemodialysis | Standard protocol | Immediate | Most effective, 25-50 mEq/hour |
Dialysis Indications:
•K+ > 6.5 mEq/L with ECG changes refractory to medical therapy
•K+ > 7.0 mEq/L
•ESRD with severe hyperkalemia
•Ongoing tissue breakdown (rhabdomyolysis, tumor lysis)
•AKI with oliguria/anuria
Chronic Hyperkalemia Management
Dietary Modification:
•Restrict potassium to < 2-3 g/day
•Avoid high-potassium foods (bananas, oranges, tomatoes, potatoes)
•Cooking methods: boiling/leaching reduces K+ content by 50-75%
Medication Adjustments:
•Consider reducing/stopping RAAS inhibitors if K+ persistently > 5.5 mEq/L
•Discontinue NSAIDs, potassium-sparing diuretics
•Review all medications for potassium content
Long-term Binder Therapy:
•Patiromer: Start 8.4 g daily, separate from other meds by 3 hours
•Sodium zirconium cyclosilicate: 5-10 g daily on non-dialysis days
•Monitoring: Check K+ weekly × 4 weeks, then monthly
CKD-Mineral and Bone Disorder (CKD-MBD) Management
KDIGO Guidelines Summary
Phosphate Management:
| CKD Stage | Target Phosphate | Intervention |
| G3a-G3b | Normal range (2.5-4.5 mg/dL) | Dietary restriction (800-1000 mg/day) |
| G4-G5 | Toward normal range | Phosphate binders + dietary restriction |
| G5D | 3.5-5.5 mg/dL | Aggressive binder therapy |
Phosphate Binder Selection:
| Binder Type | Dose | Advantages | Disadvantages |
| Calcium carbonate | 500-1000 mg TID with meals | Inexpensive, effective | Hypercalcemia risk, vascular calcification |
| Calcium acetate | 667-1334 mg TID with meals | More elemental calcium per tablet | Hypercalcemia risk |
| Sevelamer carbonate | 800-1600 mg TID with meals | No calcium load, improves lipids | Expensive, GI side effects |
| Lanthanum carbonate | 500-1000 mg TID with meals | Potent, no calcium | Expensive, GI side effects |
| Ferric citrate | 210 mg TID with meals | Treats anemia, no calcium | Expensive, GI side effects |
KDIGO Recommendations:
•Restrict calcium-based binders in patients with:
•Arterial calcification
•Adynamic bone disease
•Persistently low PTH
•Total elemental calcium intake should not exceed 1500 mg/day
Secondary Hyperparathyroidism Management
PTH Targets (KDIGO):
•CKD G3-G5: Maintain PTH in normal range or slightly elevated
•CKD G5D: PTH 2-9 times upper limit of normal (approximately 150-600 pg/mL)
Treatment Algorithm:
1.Correct Vitamin D Deficiency
•25-OH vitamin D < 30 ng/mL: Ergocalciferol or cholecalciferol
•Dosing: 50,000 IU weekly × 8-12 weeks, then monthly
2.Active Vitamin D Analogs (if PTH elevated despite correction)
•Calcitriol: 0.25-0.5 mcg daily
•Paricalcitol: 1-2 mcg daily or 2-4 mcg TIW (dialysis)
•Doxercalciferol: 2.5-10 mcg TIW (dialysis)
•Monitor calcium and phosphate weekly initially
3.Calcimimetic Therapy
•Cinacalcet: Start 30 mg daily, titrate every 2-4 weeks
•Maximum dose: 180 mg daily
•Etelcalcetide: 5 mg IV TIW (dialysis patients)
•Monitoring: PTH, calcium every 2 weeks during titration
•Side effects: Nausea (common), hypocalcemia
4.Parathyroidectomy Indications
•PTH > 800 pg/mL refractory to medical therapy
•Severe hypercalcemia (corrected Ca > 11.5 mg/dL)
•Calciphylaxis
•Intractable bone pain or fractures
Acid-Base Disorders – Advanced Management
Metabolic Acidosis in CKD
Pathophysiology:
•Decreased ammonia production
•Impaired bicarbonate reabsorption
•Reduced acid excretion
•Typically develops when GFR < 30 mL/min
Consequences of Untreated Metabolic Acidosis:
•Protein catabolism and muscle wasting
•Bone disease (buffering by bone)
•Progression of CKD
•Increased mortality
Treatment Protocol:
| Serum HCO3- | Intervention |
| 22-24 mEq/L | Consider treatment if symptomatic |
| 18-22 mEq/L | Initiate alkali therapy |
| < 18 mEq/L | Aggressive alkali therapy |
Alkali Therapy Options:
1.Sodium bicarbonate:
•Dose: 650-1300 mg (8-15 mEq) TID
•Target: HCO3- 23-29 mEq/L
•Caution: Sodium load, may worsen hypertension/edema
2.Sodium citrate:
•Dose: 10-30 mEq TID
•Better tolerated than bicarbonate
•Contraindicated with aluminum-containing antacids (increases Al absorption)
3.Fruits and vegetables:
•Alkali-producing diet
•4-5 servings/day can increase HCO3- by 2-4 mEq/L
Renal Tubular Acidosis (RTA) Classification
| Type | Defect | Urine pH | Serum K+ | Anion Gap | Causes |
| Type 1 (Distal) | H+ secretion | > 5.5 | Low | Normal | Autoimmune, amphotericin, lithium |
| Type 2 (Proximal) | HCO3- reabsorption | < 5.5 | Low | Normal | Fanconi syndrome, carbonic anhydrase inhibitors |
| Type 4 | Aldosterone deficiency/resistance | < 5.5 | High | Normal | DM, NSAIDs, ACE-I, heparin |
Type 1 RTA Treatment:
•Sodium bicarbonate 1-2 mEq/kg/day in divided doses
•Potassium citrate if hypokalemia present
•Monitor for nephrocalcinosis
Type 2 RTA Treatment:
•Sodium bicarbonate 10-15 mEq/kg/day (higher doses needed)
•Potassium supplementation essential
•Thiazide diuretics may help
Type 4 RTA Treatment:
•Fludrocortisone 0.1-0.2 mg daily (if aldosterone deficient)
•Sodium bicarbonate 1-2 mEq/kg/day
•Loop diuretics to lower potassium
•Discontinue offending medications
Hypokalemia – Detailed Evaluation
Diagnostic Algorithm
Step 1: Confirm True Hypokalemia
•Rule out pseudohypokalemia (leukocytosis > 100,000)
•Repeat measurement
Step 2: Assess Acid-Base Status
•Metabolic acidosis → GI losses, RTA, DKA
•Metabolic alkalosis → Vomiting, diuretics, hyperaldosteronism
Step 3: Measure Urine Potassium
| Urine K+ | Interpretation | Causes |
| < 20 mEq/day | Extrarenal losses | Diarrhea, vomiting, laxative abuse |
| > 20 mEq/day | Renal losses | Diuretics, RTA, hyperaldosteronism |
Step 4: Transtubular Potassium Gradient (TTKG)
Plain Text
TTKG = (Urine K+ / Plasma K+) / (Urine Osm / Plasma Osm) TTKG < 3 → Appropriate renal K+ conservation (extrarenal loss) TTKG > 4 → Inappropriate renal K+ wasting
Potassium Replacement Guidelines
Oral Replacement:
•Each 10 mEq KCl raises serum K+ by ~0.1 mEq/L
•Typical deficit: 200-400 mEq for K+ 3.0-3.5 mEq/L
•Dosing: 40-100 mEq/day in divided doses
IV Replacement:
•Peripheral line: Maximum 10 mEq/hour (20 mEq/hour with cardiac monitoring)
•Central line: Maximum 20-40 mEq/hour
•Concentration: ≤ 40 mEq/L (peripheral), ≤ 80 mEq/L (central)
Special Considerations:
•Concurrent hypomagnesemia must be corrected first
•Magnesium is required for potassium channel function
•Refractory hypokalemia often due to Mg deficiency
Magnesium Disorders
Hypomagnesemia
Causes:
•GI losses: Diarrhea, malabsorption, PPI use
•Renal losses: Diuretics, aminoglycosides, cisplatin, amphotericin
•Redistribution: Refeeding syndrome, hungry bone syndrome
Clinical Manifestations:
•Neuromuscular: Tremor, tetany, seizures
•Cardiac: Arrhythmias (torsades de pointes), prolonged QT
•Metabolic: Hypocalcemia, hypokalemia (refractory)
Treatment:
•Mild (> 1.0 mg/dL): Oral magnesium oxide 400-800 mg daily
•Severe (< 1.0 mg/dL) or symptomatic:
•Magnesium sulfate 1-2 g IV over 15 minutes (emergency)
•Maintenance: 4-6 g IV over 12-24 hours
•Monitor for hypermagnesemia (loss of DTRs, respiratory depression)
Hypermagnesemia
Causes:
•Excessive intake (antacids, laxatives, IV magnesium)
•Decreased excretion (CKD, especially GFR < 30)
Clinical Manifestations:
| Mg Level | Symptoms |
| 4-6 mg/dL | Nausea, flushing, hypotension |
| 6-8 mg/dL | Loss of deep tendon reflexes, drowsiness |
| 8-12 mg/dL | Respiratory depression, complete heart block |
| > 12 mg/dL | Cardiac arrest |
Treatment:
•Stop magnesium-containing products
•IV calcium gluconate 1-2 g (antagonizes effects)
•IV fluids + loop diuretics (if renal function adequate)
•Hemodialysis (if severe or renal failure)
Clinical Pearls for Nephrologists
1.Hyponatremia: Always calculate serum osmolality to rule out pseudohyponatremia before treatment
2.Hyperkalemia: ECG changes are more important than absolute K+ level for determining urgency
3.CKD-MBD: Start monitoring PTH and phosphate when GFR < 45 mL/min
4.Metabolic acidosis: Treat when HCO3- < 22 mEq/L in CKD to slow progression
5.Hypomagnesemia: Consider in all patients with refractory hypokalemia or hypocalcemia
6.Vaptans: Never use in hypovolemic hyponatremia – can cause severe hypotension
7.Phosphate binders: Must be taken with meals to be effective
8.RTA Type 4: Most common RTA in adults, think of it in diabetics with hyperkalemia
9.Osmotic demyelination: Risk factors include alcoholism, malnutrition, liver disease, hypokalemia
10.Cinacalcet: Start low and go slow – nausea is dose-dependent and can be minimized
References
1.Sterns RH. Treatment of severe hyponatremia. Clin J Am Soc Nephrol. 2018;13(4):641-649.
2.European Clinical Practice Guideline on the management of hyponatraemia. Nephrol Dial Transplant. 2014;29 Suppl 2:i1-i39.
3.KDIGO 2017 Clinical Practice Guideline Update for the Diagnosis, Evaluation, Prevention, and Treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2017;7(1):1-59.
4.Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017;84(12):934-942.
5.Kovesdy CP. Management of hyperkalaemia in chronic kidney disease. Nat Rev Nephrol. 2014;10(11):653-662.
6.Wesson DE, Mathur V, Tangri N, et al. Long-term safety and efficacy of veverimer in patients with metabolic acidosis in chronic kidney disease: a multicentre, randomised, blinded, placebo-controlled, 40-week extension. Lancet. 2019;394(10196):396-406.
7.Palmer BF, Clegg DJ. Diagnosis and treatment of hyperkalemia. Cleve Clin J Med. 2017;84(12):934-942.
8.Chifu I, Seidel V, Zopf K, et al. Treatment of symptomatic hyponatremia with hypertonic saline: a single-center experience. BMC Nephrol. 2021;22(1):150.
🎯 MULTIPLE CHOICE QUESTIONS
Question 1
A 68-year-old man with SCLC presents with serum sodium of 118 mEq/L, serum osmolality 245 mOsm/kg, urine osmolality 520 mOsm/kg, and urine sodium 85 mEq/L. He is confused but arousable. You initiate 3% saline at 30 mL/hour. Six hours later, his sodium is 126 mEq/L and he is alert.
What is the MOST appropriate next step?
A) Continue 3% saline at the same rate to reach target of 135 mEq/L
B) Stop hypertonic saline and monitor sodium every 2 hours
C) Decrease 3% saline rate to 15 mL/hour
D) Administer desmopressin 2 mcg IV and give D5W
E) Switch to 0.9% normal saline”
Correct Answer: D
Explanation: The patient’s sodium increased by 8 mEq/L in 6 hours, which exceeds the safe correction limit of 6 mEq/L per 6 hours. This puts him at risk for osmotic demyelination syndrome (ODS). The most appropriate action is to administer desmopressin (DDAVP) to induce water retention and give hypotonic fluids (D5W) to lower the sodium. Simply stopping hypertonic saline may not be sufficient to prevent overcorrection. The goal is to keep total correction under 8 mEq/L in 24 hours for chronic hyponatremia.”,
Question 2
A 45-year-old woman on hemodialysis presents with serum potassium of 7.8 mEq/L. ECG shows widened QRS complexes. You administer calcium gluconate 2 grams IV, insulin 10 units with dextrose, and albuterol nebulizer. Thirty minutes later, repeat potassium is 7.2 mEq/L and QRS remains wide.
Which of the following is the MOST appropriate next step?”,
A) Repeat calcium gluconate 2 grams IV immediately
B) Administer sodium bicarbonate 100 mEq IV
C) Give sodium polystyrene sulfonate (Kayexalate) 30 grams PO
D) Arrange emergent hemodialysis
E) Administer patiromer 8.4 grams PO”
Correct Answer: D
Explanation: This patient has severe hyperkalemia with persistent ECG changes (wide QRS) despite initial medical management. Hemodialysis is the most effective method to rapidly remove potassium from the body, lowering K+ by 1-1.5 mEq/L per hour. Repeating calcium would only temporarily stabilize the cardiac membrane without lowering potassium. Sodium bicarbonate is only effective if acidosis is present. Kayexalate and patiromer work too slowly (hours to days) for this emergency situation. In dialysis patients with life-threatening hyperkalemia and persistent ECG changes, emergent dialysis is the definitive treatment.”,
Question 3
A 62-year-old diabetic man with CKD stage 4 (eGFR 22 mL/min) on lisinopril presents with potassium 6.2 mEq/L and bicarbonate 18 mEq/L. Urine pH is 5.2.
On Exam He has Lower extremity edema +2 bilateral
Which type of renal tubular acidosis is MOST likely, and what is the best initial management?
A) Type 1 RTA; initiate sodium bicarbonate 650 mg TID and potassium citrate
B) Type 2 RTA; initiate high-dose sodium bicarbonate 1300 mg TID and thiazide diuretic
C) Type 4 RTA; discontinue lisinopril and initiate loop diuretic
D) Type 4 RTA; initiate fludrocortisone 0.1 mg daily
E) Mixed RTA; initiate sodium bicarbonate and patiromer
Correct Answer: C
Explanation: This patient has Type 4 RTA, characterized by hyperkalemia, metabolic acidosis with normal anion gap, and ability to acidify urine (pH < 5.5). Type 4 RTA is the most common RTA in adults and is frequently caused by hyporeninemic hypoaldosteronism in diabetics, often exacerbated by ACE inhibitors. The best initial management is to discontinue the ACE inhibitor (lisinopril) and start a loop diuretic to enhance potassium excretion. Fludrocortisone can be used if aldosterone deficiency is confirmed, but stopping the offending agent is the first step. Types 1 and 2 RTA present with hypokalemia, not hyperkalemia.
Question 4
A 55-year-old woman with CKD G4 has the following labs: Ca 8.2 mg/dL, PO4 6.8 mg/dL, PTH 425 pg/mL (normal 10-65), 25-OH vitamin D 18 ng/mL. She is currently on calcium carbonate 1000 mg TID with meals.
What is the MOST appropriate next step in management?
A) Increase calcium carbonate to 1500 mg TID
B) Switch to sevelamer 800 mg TID and start ergocalciferol 50,000 IU weekly
C) Add calcitriol 0.25 mcg daily
D) Start cinacalcet 30 mg daily
E) Refer for parathyroidectomy
Correct Answer B
Explanation: This patient has secondary hyperparathyroidism with vitamin D deficiency and hyperphosphatemia. The most appropriate approach is to:
(1) switch from calcium-based to non-calcium-based phosphate binder (sevelamer) because she’s hyperphosphatemic and at risk for vascular calcification with continued calcium supplementation, and
(2) correct vitamin D deficiency with ergocalciferol before starting active vitamin D analogs. Increasing calcium carbonate would worsen hyperphosphatemia and increase calcium-phosphate product. Starting calcitriol without correcting 25-OH vitamin D deficiency is premature. Cinacalcet is third-line therapy. Parathyroidectomy is reserved for refractory cases with PTH > 800 pg/mL.”,
Question 5
A 70-year-old man on hemodialysis for 3 years develops severe, painful skin lesions with violaceous discoloration and necrosis on his abdomen and thighs. Labs show Ca 9.8 mg/dL, PO4 7.2 mg/dL, PTH 890 pg/mL. Skin biopsy confirms calciphylaxis.
Which of the following is the MOST appropriate management strategy?”,
A) Intensify phosphate binder therapy with calcium acetate
B) Start cinacalcet, switch to non-calcium phosphate binders, and increase dialysis frequency
C) Initiate high-dose vitamin D therapy with paricalcitol
D) Urgent parathyroidectomy
E) Hyperbaric oxygen therapy
Correct Answer A
Explanation: Calciphylaxis (calcific uremic arteriolopathy) is a life-threatening complication with 50-80% mortality. Management includes: (1) starting cinacalcet to lower PTH, (2) switching to non-calcium-based phosphate binders (sevelamer or lanthanum) to avoid calcium load, (3) intensifying dialysis to improve calcium-phosphate removal, and (4) wound care. Calcium-based binders are contraindicated as they worsen tissue calcification. Active vitamin D analogs can increase calcium-phosphate product and should be used cautiously or held. Parathyroidectomy may be considered for refractory hyperparathyroidism but is not first-line for calciphylaxis. Sodium thiosulfate IV is also an emerging therapy.”,
Question 6
A 58-year-old woman with CKD G3b (eGFR 38 mL/min) has bicarbonate of 19 mEq/L. You initiate sodium bicarbonate 650 mg TID. Three months later, her bicarbonate is 24 mEq/L, but she has gained 4 kg and her blood pressure increased from 128/78 to 148/92 mmHg.
What is the MOST appropriate modification to her therapy?
A) Discontinue sodium bicarbonate due to volume overload
B) Switch to sodium citrate solution
C) Reduce sodium bicarbonate to 650 mg daily
D) Continue sodium bicarbonate and add a thiazide diuretic
E) Switch to dietary alkali therapy with increased fruit and vegetable intake
Correct Answer: E
Explanation :The patient has achieved target bicarbonate but developed volume overload and hypertension from the sodium load in sodium bicarbonate (each gram provides 12 mEq of sodium). The best approach is to switch to dietary alkali therapy (4-5 servings of fruits and vegetables daily), which can increase bicarbonate by 2-4 mEq/L without sodium load. This maintains the benefits of treating metabolic acidosis (slowing CKD progression, preserving muscle mass) while avoiding volume-related complications. Sodium citrate contains similar sodium content. Simply reducing the dose may lead to inadequate acidosis treatment. Discontinuing therapy entirely would lose the renoprotective benefits. Adding a diuretic treats the symptom but not the cause.
Goraya N, Simoni J, Jo CH, Wesson DE. A comparison of treating metabolic acidosis in CKD stage 4 hypertensive kidney disease with fruits and vegetables or sodium bicarbonate. Clin J Am Soc Nephrol. 2013;8(3):371–381.
de Brito-Ashurst I, et al. Bicarbonate supplementation slows progression of CKD and improves nutritional status. J Am Soc Nephrol. 2009;20(9):2075–2084.
Banerjee T, Crews DC, Wesson DE. Dietary acid load and chronic kidney disease among adults in the United States. Kidney Int. 2015;88(1):226–233.
Question 7
A 42-year-old woman with a history of Sjögren syndrome presents with fatigue and bone pain. Labs show: Na 138 mEq/L, K 2.8 mEq/L, Cl 112 mEq/L, HCO3 16 mEq/L, anion gap 10, urine pH 6.5, urine anion gap +15. Serum calcium is 8.9 mg/dL. Renal ultrasound shows bilateral nephrocalcinosis.
What is the MOST likely diagnosis and appropriate treatment?
A) Type 2 (proximal) RTA; sodium bicarbonate 10-15 mEq/kg/day
B) Type 1 (distal) RTA; sodium bicarbonate 1-2 mEq/kg/day and potassium citrate
C) Type 4 RTA; fludrocortisone and loop diuretic
D) Fanconi syndrome; high-dose alkali therapy and phosphate supplementation
E) Diarrhea-induced metabolic acidosis; loperamide and oral rehydration
Correct Answer: B
Explanation : This patient has Type 1 (distal) RTA, characterized by: (1) inability to acidify urine (pH > 5.5) despite systemic acidosis, (2) hypokalemia, (3) positive urine anion gap (indicating impaired NH4+ excretion), (4) nephrocalcinosis, and (5) association with autoimmune diseases like Sjögren syndrome. The defect is in H+ secretion in the collecting duct. Treatment requires lower doses of alkali (1-2 mEq/kg/day) compared to Type 2 RTA, plus potassium supplementation (citrate preferred as it also provides alkali. Type 2 RTA can acidify urine when bicarbonate is low. Type 4 RTA presents with hyperkalemia. The positive urine anion gap rules out GI losses.
Question 8:
A 65-year-old man with heart failure (EF 25%) is admitted with volume overload. Serum sodium is 128 mEq/L. Despite aggressive diuresis with furosemide, his sodium drops to 124 mEq/L over 48 hours. Urine sodium is 75 mEq/L, urine osmolality 450 mOsm/kg.
Which medication would be MOST appropriate to add?
A) Demeclocycline 300 mg BID
B) Tolvaptan 15 mg daily”,
C) Conivaptan 20 mg IV loading dose
D) Hypertonic 3% saline infusion”,
E) Urea 30 grams daily
Correct Answer : B
Explanation: This patient has hypervolemic hyponatremia due to heart failure with paradoxical worsening despite diuresis (high urine sodium indicates effective diuresis, but non-osmotic ADH release persists). Tolvaptan, a selective V2 receptor antagonist (vaptans), is FDA-approved for hypervolemic and euvolemic hyponatremia and promotes aquaresis (free water excretion) without electrolyte loss. It’s particularly useful in heart failure patients. Conivaptan is IV-only and approved for short-term hospital use. Demeclocycline works but has slower onset and more side effects. Hypertonic saline would worsen volume overload. Urea is effective but poorly tolerated. The SALT trials demonstrated tolvaptan’s efficacy in raising sodium in heart failure patients.
Question 9
A 52-year-old man with CKD G5 on hemodialysis has persistent hyperphosphatemia (PO4 7.8 mg/dL) despite sevelamer 1600 mg TID with meals. His calcium is 9.2 mg/dL and PTH is 320 pg/mL. Dialysis adequacy (Kt/V) is 1.5.
What is the MOST appropriate next step?”,
A) Add lanthanum carbonate 500 mg TID to sevelamer
B) Switch from sevelamer to calcium acetate for better phosphate binding
C) Increase dialysis time from 3.5 to 4 hours per session
D) Add niacin 500 mg BID to reduce intestinal phosphate absorption
E) Strict dietary phosphate restriction to < 600 mg/day
Correct Answer A
Explanation: This patient has refractory hyperphosphatemia despite maximum-dose sevelamer and adequate dialysis (Kt/V > 1.2). The most appropriate approach is to add a second phosphate binder with a different mechanism. Lanthanum carbonate is a potent non-calcium binder that can be combined with sevelamer for additive effect. Switching to calcium acetate risks hypercalcemia and vascular calcification, especially with calcium already at 9.2 mg/dL. While increasing dialysis time can help, the Kt/V is already adequate. Niacin (nicotinic acid) can reduce phosphate absorption but causes significant flushing and is not first-line. Dietary restriction alone is insufficient when already on maximum binder therapy. Combination binder therapy is evidence-based for refractory cases.
Question 10
A 48-year-old woman presents to the ED with severe muscle weakness and difficulty walking. ECG shows prominent U waves and flattened T waves. Serum potassium is 1.9 mEq/L, magnesium 1.1 mg/dL (normal 1.7-2.2), bicarbonate 32 mEq/L, blood pressure 168/98 mmHg. Urine potassium is 35 mEq/L. Despite IV potassium chloride 40 mEq over 4 hours, repeat potassium is 2.1 mEq/L.
What is the MOST likely diagnosis?
A) Gitelman syndrome
B) Bartter syndrom
C) Primary hyperaldosteronism
D) Liddle syndrome
E) Apparent mineralocorticoid excess
Correct Answer: C
Explanation:
Despite hypertension, the combination of hypokalemia, metabolic alkalosis, and hypomagnesemia with poor K response suggests Gitelman-like physiology.
But true Gitelman patients are typically normotensive or mildly hypotensive.
So — could she have two processes? Unlikely; this question is testing recognition of patterns.
The persistence of renal K+ wasting despite supplementation is classically due to magnesium deficiency, which must be corrected first for K⁺ to rise.
However, when we must choose a single most likely diagnosis from the list, the hypertension overrides everything.
High BP → rules out Gitelman and Bartter.
High urine K and metabolic alkalosis → renal K loss via ENaC or aldosterone.
Response to KCl poor → consistent with continued mineralocorticoid activity.
Magnesium loss → not typical but may occur secondary to diuretics or other factors, not part of diagnosis per se.
Therefore, the most likely cause is Primary Hyperaldosteronism.