Introduction
The kidneys are remarkable organs that perform essential functions in maintaining homeostasis, including waste excretion, electrolyte balance, acid-base regulation, blood pressure control, and hormone production. A thorough understanding of renal anatomy is fundamental to comprehending kidney physiology, pathophysiology, and clinical nephrology. This chapter provides an in-depth exploration of renal anatomy, from macroscopic structure to microscopic architecture, with emphasis on clinically relevant anatomical relationships and functional correlations.
The kidneys are paired retroperitoneal organs that receive approximately 20-25% of cardiac output (approximately 1,200 mL/min), despite representing less than 0.5% of total body weight. This remarkable blood flow reflects their critical role in filtering blood and maintaining internal homeostasis. Each kidney contains approximately 1 to 1.5 million nephrons, the functional units responsible for urine formation and regulation of body fluid composition.
Gross Anatomy of the Kidney
Location and Positioning
The kidneys are bean-shaped retroperitoneal organs located in the posterior abdomen, positioned lateral to the vertebral column between the T12 and L3 vertebrae. The right kidney typically sits slightly lower than the left kidney due to displacement by the liver, which occupies a significant portion of the right upper abdomen. Both kidneys lie immediately inferior to their respective adrenal (suprarenal) glands.
The upper pole of each kidney is covered by the diaphragm and is frequently crossed by the 12th rib, making this region vulnerable to injury during trauma. The lower poles are positioned anterior to the psoas major muscle medially and the quadratus lumborum muscle laterally. The kidneys are aligned slightly obliquely, parallel to the ipsilateral psoas muscle, with the lower poles being more lateral, anterior, and farther apart than the upper poles.
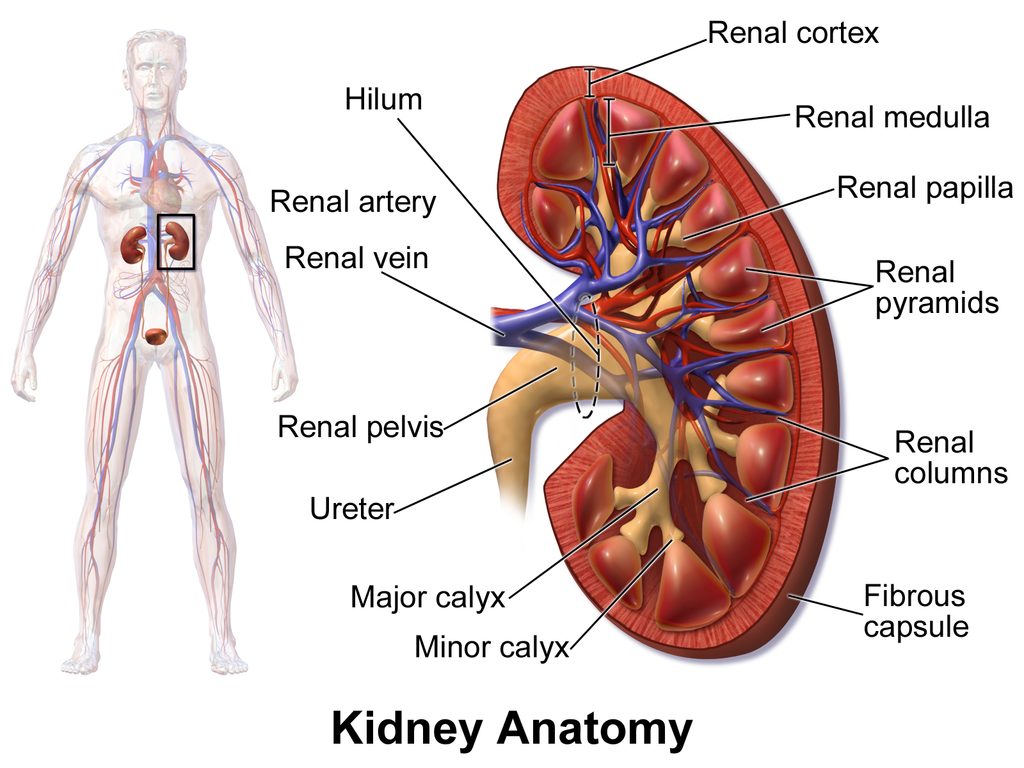
Figure 1: Comprehensive Kidney Anatomy
Image shows the internal structure of the kidney including the hilum, renal artery, renal vein, renal cortex, renal medulla, renal papilla, renal pyramids, renal columns, renal pelvis, ureter, major calyx, minor calyx, and fibrous capsule. Source: Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1(2). DOI:10.15347/wjm/2014.010. Licensed under CC BY 3.0.
Size and Dimensions
The kidneys exhibit considerable consistency in size and proportions across individuals, though dimensions correlate positively with body surface area, height, and weight. Typical measurements include:
•Length: 10-12 cm (approximately the size of a closed fist)
•Width: 5-7 cm
•Thickness: 3-5 cm
•Weight: 160-162 g in males, 135-136 g in females
The left kidney is generally slightly larger and approximately 10 g heavier than the right kidney. Kidney size decreases with age due to progressive loss of renal parenchyma, a process that accelerates after age 50 and contributes to the age-related decline in glomerular filtration rate (GFR).
Anatomical Relations
Understanding the anatomical relationships of the kidneys is essential for interpreting imaging studies and planning surgical interventions.
Right Kidney:
•Anterior: Liver (separated by hepatorenal recess), second part of duodenum, ascending colon
•Posterior: Diaphragm (upper third), 12th rib, psoas major, quadratus lumborum, transversus abdominis
•Medial: Inferior vena cava, right adrenal gland
Left Kidney:
•Anterior: Stomach (superomedial surface), spleen (connected via splenorenal ligament), tail of pancreas (may extend to cover hilum), descending colon
•Posterior: Diaphragm (upper third), 11th and 12th ribs, psoas major, quadratus lumborum, transversus abdominis
•Medial: Abdominal aorta, left adrenal gland
The colon is positioned anterior to both kidneys, an important consideration during percutaneous renal procedures to avoid colonic injury.
Renal Capsule and Surrounding Structures
Each kidney is enclosed by multiple layers of tissue that provide support, protection, and compartmentalization:
1.Renal capsule: A tough fibrous layer directly adherent to the kidney surface
2.Perinephric (perirenal) fat: Adipose tissue surrounding the capsule, thickest along the kidney borders
3.Renal fascia:
•Gerota fascia (anterior renal fascia)
•Zuckerkandl fascia (posterior renal fascia)
•These fasciae fuse laterally and superiorly but remain open inferiorly, allowing potential spread of infection or hemorrhage
4.Paranephric (pararenal) fat: Adipose tissue external to the renal fascia
The term “Gerota fascia” is frequently used in clinical practice to refer collectively to both the anterior and posterior renal fasciae. The perinephric fat extends into the renal sinus at the hilum, providing cushioning for the renal vessels and collecting system.
Renal Hilum
The renal hilum is a vertical slit located on the medial concave border of each kidney, serving as the entry and exit point for renal vessels, nerves, lymphatics, and the renal pelvis. The structures within the hilum are arranged in a consistent anatomical relationship, from anterior to posterior:
1.Renal vein (most anterior)
2.Renal artery (middle)
3.Renal pelvis (most posterior)
This arrangement can be remembered by the mnemonic “VAP” (Vein, Artery, Pelvis) from anterior to posterior. Understanding this relationship is crucial during surgical dissection and vascular access procedures.
Internal Architecture
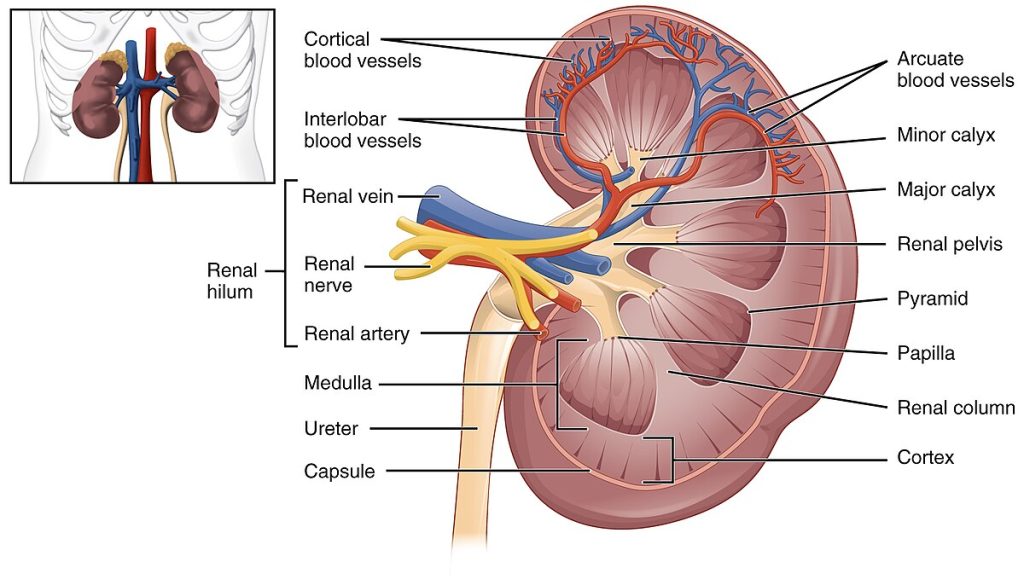
Figure 2: Internal Architecture of the Kidney
Detailed cross-section showing cortical and medullary regions, renal pyramids, renal columns, and collecting system. Source: OpenStax Anatomy & Physiology. Licensed under CC BY 3.0.
Renal Parenchyma
Upon sectioning a kidney in the coronal plane, two distinct regions of renal parenchyma are visible: the cortex and the medulla. These regions differ in color, texture, and function, reflecting their distinct roles in urine formation.
Renal Cortex
The renal cortex forms the outer portion of the kidney parenchyma and appears reddish-brown due to its rich vascular supply. The cortex contains the majority of nephron components, including:
•Renal corpuscles (glomeruli and Bowman capsules)
•Proximal convoluted tubules
•Distal convoluted tubules
•Cortical collecting ducts
•Peritubular capillary networks
The cortex can be subdivided into two regions:
1.Cortical mantle: The peripheral layer of cortical tissue that covers the base of each renal pyramid
2.Renal columns (columns of Bertin): Extensions of cortical tissue that project inward between adjacent renal pyramids, extending from the mantle toward the renal sinus
The cortex receives approximately 90% of renal blood flow, reflecting its high metabolic activity and filtration function.
Renal Medulla
The renal medulla lies internal to the cortex and appears paler due to lower vascularity. The medulla is organized into 8-18 conical structures called renal pyramids, which contain:
•Loops of Henle (descending and ascending limbs)
•Medullary collecting ducts
•Vasa recta (specialized capillaries)
The apex of each pyramid, called the renal papilla, projects into a minor calyx. The papillary surface contains multiple small openings (the area cribrosa) through which urine drains from the papillary ducts into the collecting system.
Medullary rays are striations visible within the cortex that represent extensions of medullary tissue containing straight portions of tubules (proximal straight tubules, distal straight tubules, and collecting ducts) that project from the pyramids into the overlying cortex.
Renal Lobe
A renal lobe is the functional anatomical unit of the kidney, consisting of a single renal pyramid together with its overlying cortical tissue and associated renal column. Each kidney typically contains 8-9 renal lobes, though this number can vary from 5 to 14. Each lobe is drained by a single renal papilla.
Collecting System (Pyelocalyceal System)
The renal collecting system receives urine from the nephrons and conveys it to the ureter. This system is lined by transitional epithelium (urothelium) and consists of the following components, arranged from peripheral to central:
1.Minor calyces: Cup-shaped structures that receive urine from individual renal papillae. Most kidneys contain 7-9 minor calyces (range 4-13).
2.Major calyces: Formed by the convergence of 2-3 minor calyces. Typically, there are 2-3 major calyces per kidney (upper, middle, and lower, or upper and lower).
3.Renal pelvis: A funnel-shaped structure formed by the union of major calyces. The renal pelvis represents the expanded upper end of the ureter.
4.Ureter: The muscular tube that conveys urine from the renal pelvis to the bladder.
Anatomical Variations
Significant variation exists in collecting system anatomy:
•Simple calyces: A minor calyx that drains a single papilla (most common)
•Compound calyces: A minor calyx that drains 2-3 fused papillae, more common in the upper pole
•Extrarenal pelvis: The renal pelvis lies predominantly outside the renal sinus (facilitates percutaneous access)
•Intrarenal pelvis: The renal pelvis lies predominantly within the renal sinus (may complicate stone removal)
These anatomical variations have important implications for urological procedures, including ureteroscopy, percutaneous nephrolithotomy, and extracorporeal shockwave lithotripsy.
The Nephron: Functional Unit of the Kidney
The nephron is the structural and functional unit of the kidney responsible for filtering blood and forming urine. Each adult kidney contains approximately 1 to 1.5 million nephrons, with considerable variation between individuals (range 200,000 to over 2.5 million). Individuals born with fewer nephrons have increased susceptibility to chronic kidney disease and kidney failure later in life.
Nephron Structure
Each nephron consists of two main components: the renal corpuscle and the renal tubule.
1. Renal Corpuscle
The renal corpuscle is the site of blood filtration and consists of two structures:
A. Glomerulus
•A tuft of specialized capillaries supplied by an afferent arteriole and drained by an efferent arteriole
•Capillary endothelium is fenestrated (contains pores 70-100 nm in diameter)
•Site of ultrafiltration of blood plasma
•Approximately 180 liters of fluid are filtered daily through glomerular capillaries
B. Bowman Capsule (Glomerular Capsule)
•A double-layered epithelial cup that surrounds the glomerulus
•Parietal layer: Simple squamous epithelium forming the outer wall
•Visceral layer: Specialized epithelial cells called podocytes that directly contact glomerular capillaries
•Bowman space (urinary space): The cavity between the two layers where filtrate collects
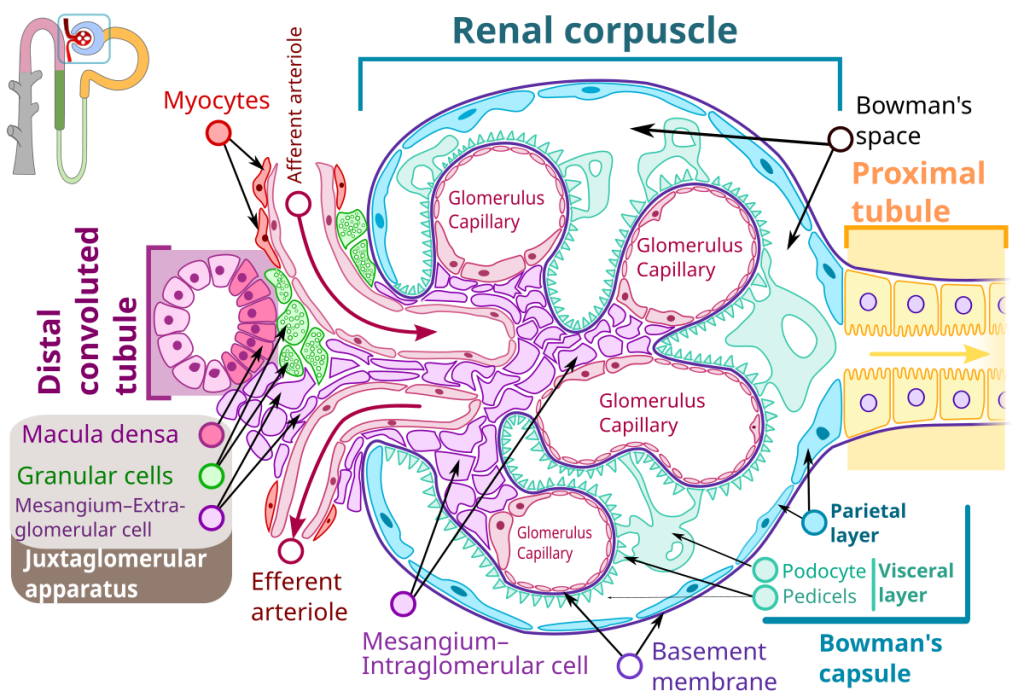
Figure 3: Renal Corpuscle Structure with Juxtaglomerular Apparatus
Detailed diagram showing the glomerulus, Bowman’s capsule, podocytes (visceral layer), parietal layer, afferent and efferent arterioles, proximal tubule, distal tubule, macula densa, granular cells (juxtaglomerular cells), and mesangial cells. The juxtaglomerular apparatus is highlighted showing the relationship between the macula densa of the distal tubule and the granular cells of the afferent arteriole. Source: Wikimedia Commons (derivative work by M•Komorniczak based on Shypoetess). Licensed under CC BY-SA 4.0.
2. Renal Tubule
The renal tubule is a long, coiled structure that processes the glomerular filtrate through selective reabsorption and secretion. The tubule consists of several functionally distinct segments:
A. Proximal Tubule
The proximal tubule is the longest and most metabolically active segment of the nephron, responsible for reabsorbing approximately 65-70% of filtered sodium and water, along with nearly all filtered glucose, amino acids, and bicarbonate.
•Proximal convoluted tubule (PCT): The initial coiled portion located in the cortex
•Proximal straight tubule (thick descending limb): The straight portion that descends into the medulla
The proximal tubule epithelium features a prominent brush border (microvilli) on the luminal surface, which dramatically increases surface area for reabsorption. These cells are rich in mitochondria to support active transport processes.
B. Loop of Henle
The loop of Henle creates and maintains the medullary osmotic gradient essential for urine concentration. It consists of:
•Thin descending limb: Highly permeable to water but relatively impermeable to solutes; water exits passively into the hypertonic medullary interstitium
•Thin ascending limb: Present only in juxtamedullary nephrons; impermeable to water but permeable to sodium and chloride
•Thick ascending limb (TAL): Actively transports sodium, potassium, and chloride via the Na-K-2Cl cotransporter (target of loop diuretics); impermeable to water, creating “dilute urine”
C. Distal Tubule
The distal tubule fine-tunes electrolyte balance and acid-base homeostasis under hormonal regulation.
•Distal convoluted tubule (DCT): Located in the cortex; site of Na-Cl cotransporter (target of thiazide diuretics); regulated by aldosterone and parathyroid hormone
•Connecting tubule: Transitional segment between DCT and collecting duct
D. Collecting Duct
The collecting duct is the final site of urine modification and is responsible for final urine concentration under the influence of antidiuretic hormone (ADH/vasopressin).
•Cortical collecting duct: Begins in the cortex; contains principal cells (respond to aldosterone and ADH) and intercalated cells (regulate acid-base balance)
•Medullary collecting duct: Descends through the medulla; final site of water reabsorption in response to ADH
•Papillary duct (duct of Bellini): Terminal portion that opens at the renal papilla
Types of Nephrons
Nephrons are classified based on the location of their renal corpuscles:
1. Cortical Nephrons (85%)
•Renal corpuscles located in the outer cortex
•Short loops of Henle that extend only to the outer medulla
•Primarily involved in filtration and reabsorption
•Supplied by peritubular capillaries
2. Juxtamedullary Nephrons (15%)
•Renal corpuscles located at the corticomedullary junction
•Long loops of Henle that extend deep into the inner medulla
•Critical for establishing and maintaining the medullary osmotic gradient
•Supplied by vasa recta (long, straight capillaries)
•Essential for urine concentration
Renal Vasculature
The kidneys have a unique and highly specialized vascular system designed to support their filtration function while maintaining precise regulation of blood flow and pressure.
Arterial Supply
The renal arteries arise from the abdominal aorta at the level of L1-L2 vertebrae, just below the origin of the superior mesenteric artery. The right renal artery is longer than the left and passes posterior to the inferior vena cava.
The arterial blood supply follows a hierarchical branching pattern:
1.Renal artery → enters at the hilum
2.Segmental arteries (5 segments: apical, upper, middle, lower, posterior)
3.Interlobar arteries → ascend between renal pyramids in the renal columns
4.Arcuate arteries → arch over the bases of the pyramids at the corticomedullary junction
5.Interlobular arteries (cortical radial arteries) → project into the cortex perpendicular to the arcuate arteries
6.Afferent arterioles → supply individual glomeruli
7.Glomerular capillaries → site of filtration
8.Efferent arterioles → drain glomeruli
After the glomerulus, the efferent arterioles give rise to two distinct capillary networks:
•Peritubular capillaries: Surround cortical nephron tubules; facilitate reabsorption and secretion
•Vasa recta: Long, straight capillaries that descend into the medulla alongside juxtamedullary nephron loops of Henle; essential for maintaining the medullary osmotic gradient through countercurrent exchange
Clinical Significance: End Arteries and Brödel Line
The interlobar and arcuate arteries function as end arteries with minimal collateral circulation. Occlusion of these vessels results in segmental infarction of the kidney. This anatomical feature has important implications:
•Renal infarction from emboli typically produces wedge-shaped infarcts
•Surgical planning must account for segmental vascular anatomy
•The Brödel line (also called the avascular plane of Brödel) is a relatively avascular longitudinal zone between the anterior and posterior segmental arterial territories, typically located slightly posterior to the lateral convex border of the kidney. This plane is the preferred entry point for percutaneous nephrolithotomy to minimize bleeding risk.
Venous Drainage
The venous drainage of the kidney follows a pattern that mirrors the arterial supply, but in reverse:
1.Peritubular capillaries and vasa recta → drain into
2.Interlobular veins (cortical radial veins) →
3.Arcuate veins →
4.Interlobar veins →
5.Renal vein → exits at the hilum and drains into the inferior vena cava
Anatomical Asymmetry of Renal Veins
Significant anatomical differences exist between the left and right renal veins:
Left Renal Vein:
•Length: 7-10 cm (longer)
•Crosses anterior to the aorta to reach the IVC
•Receives tributaries from:
•Left adrenal (suprarenal) vein
•Left gonadal vein (testicular or ovarian)
•Lumbar veins (variable)
•May be compressed between the aorta and superior mesenteric artery (nutcracker syndrome)
Right Renal Vein:
•Length: 2-4 cm (shorter)
•Courses directly to the IVC without crossing the aorta
•Typically receives no major tributaries
Clinical Implications:
•The longer left renal vein makes the left kidney preferred for living donor transplantation, as it provides greater length for vascular anastomosis
•Left-sided varicocele (85-90% of cases) is common and usually benign due to drainage of the left gonadal vein into the left renal vein at a right angle
•Isolated right varicocele is a red flag for malignancy (renal cell carcinoma with tumor thrombus or IVC obstruction) and warrants urgent imaging
The Juxtaglomerular Apparatus
The juxtaglomerular apparatus (JGA) is a specialized structure located at the vascular pole of the renal corpuscle where the distal tubule comes into close contact with the afferent and efferent arterioles. The JGA plays a critical role in regulating glomerular filtration rate, renal blood flow, and systemic blood pressure through two key mechanisms: tubuloglomerular feedback and renin secretion.
Components of the Juxtaglomerular Apparatus
The JGA consists of three main cellular components (see Figure 3):
1. Macula Densa
•Specialized epithelial cells in the wall of the thick ascending limb of the loop of Henle at the point where it contacts the glomerular arterioles
•Cells are taller and more densely packed than surrounding tubular epithelium
•Function as chemoreceptors that sense sodium chloride concentration in the tubular fluid
•Lack a brush border, allowing direct contact with tubular fluid
2. Granular Cells (Juxtaglomerular Cells)
•Modified smooth muscle cells located in the wall of the afferent arteriole (and to a lesser extent, the efferent arteriole)
•Contain renin-secreting granules
•Function as mechanoreceptors sensitive to changes in afferent arteriolar pressure
•Synthesize, store, and release renin in response to multiple stimuli
3. Extraglomerular Mesangial Cells (Lacis Cells)
•Located in the triangular space between the afferent arteriole, efferent arteriole, and macula densa
•Continuous with intraglomerular mesangial cells
•Function incompletely understood; may play a role in cell-to-cell communication and structural support
Functions of the Juxtaglomerular Apparatus
A. Tubuloglomerular Feedback (Autoregulation)
Tubuloglomerular feedback is a local negative feedback mechanism that maintains stable GFR despite fluctuations in systemic blood pressure.
Mechanism:
1.Increased GFR → increased NaCl delivery to the macula densa
2.Macula densa senses elevated NaCl concentration
3.Macula densa releases ATP and adenosine
4.Adenosine causes vasoconstriction of the afferent arteriole
5.Decreased glomerular capillary pressure → decreased GFR (returns to baseline)
This mechanism operates effectively when mean arterial pressure is between 80-180 mmHg, maintaining relatively constant GFR and renal blood flow.
B. Renin-Angiotensin-Aldosterone System (RAAS)
The JGA is the primary site of renin production and secretion, initiating the RAAS cascade that regulates blood pressure and sodium balance.
Stimuli for Renin Release:
1.Decreased renal perfusion pressure (detected by baroreceptors in afferent arteriole)
2.Decreased NaCl delivery to macula densa (detected by macula densa chemoreceptors)
3.Sympathetic stimulation via β1-adrenergic receptors on granular cells
RAAS Cascade:
1.Renin (released by granular cells) → cleaves angiotensinogen (produced by liver) to angiotensin I
2.Angiotensin-converting enzyme (ACE, primarily in lungs) → converts angiotensin I to angiotensin II
3.Angiotensin II effects:
•Vasoconstriction (increases blood pressure)
•Stimulates aldosterone release (increases sodium and water retention)
•Stimulates ADH release (increases water retention)
•Increases proximal tubule sodium reabsorption
•Stimulates thirst
Clinical Significance:
•ACE inhibitors and angiotensin receptor blockers (ARBs) are cornerstone therapies for hypertension, heart failure, and chronic kidney disease
•Disruption of JGA function contributes to renovascular hypertension
•The JGA is the target of several antihypertensive medications
Glomerular Filtration Barrier
The glomerular filtration barrier is a highly specialized structure that permits the passage of water and small solutes while restricting the filtration of larger molecules, particularly proteins and blood cells. This selective permeability is achieved through a three-layered barrier that provides both size selectivity and charge selectivity.
Three Layers of the Filtration Barrier
1. Fenestrated Endothelium
The innermost layer consists of the capillary endothelial cells that line the glomerular capillaries.
•Fenestrations (pores): 70-100 nm in diameter, arranged in a sieve-like pattern
•Glycocalyx: A negatively charged layer of glycoproteins and proteoglycans coating the endothelial surface
•Function: Prevents passage of blood cells; contributes to charge selectivity by repelling negatively charged proteins (e.g., albumin)
2. Glomerular Basement Membrane (GBM)
The middle layer is a thick (300-350 nm), acellular extracellular matrix produced by both endothelial cells and podocytes.
Composition:
•Type IV collagen: Provides structural framework (primarily α3, α4, α5 chains)
•Laminin: Glycoprotein that provides adhesion
•Proteoglycans (heparan sulfate): Negatively charged molecules that contribute to charge selectivity
•Nidogen and entactin: Bridging molecules
Layers of GBM:
•Lamina rara interna: Adjacent to endothelium
•Lamina densa: Central dense layer
•Lamina rara externa: Adjacent to podocytes
Function: Primary size-selective barrier; restricts passage of molecules >69 kDa (albumin is 69 kDa); negative charge repels anionic proteins
3. Podocyte Layer (Visceral Epithelium)
The outermost layer consists of highly specialized epithelial cells called podocytes that wrap around the glomerular capillaries.
Structure:
•Cell body: Contains the nucleus and organelles
•Primary processes: Major extensions from the cell body
•Foot processes (pedicels): Interdigitating finger-like projections that rest on the GBM
•Filtration slits (slit diaphragms): 25-60 nm gaps between adjacent foot processes, bridged by nephrin and other slit diaphragm proteins
Key Proteins:
•Nephrin: Transmembrane protein that forms the structural backbone of the slit diaphragm (zipper-like configuration)
•Podocin: Anchors nephrin to the podocyte cytoskeleton
•CD2AP: Adaptor protein linking nephrin to the actin cytoskeleton
•α-actinin-4: Cytoskeletal protein maintaining foot process structure
Function: Final barrier to protein filtration; maintains structural integrity of the filtration barrier; podocyte injury is the most common cause of nephrotic-range proteinuria
Size and Charge Selectivity
The glomerular filtration barrier exhibits both size and charge selectivity:
Size Selectivity:
•Molecules <20 kDa: Freely filtered (e.g., inulin, creatinine, urea)
•Molecules 20-69 kDa: Partially filtered (filtration decreases with increasing size)
•Molecules >69 kDa: Minimally filtered (e.g., albumin, immunoglobulins)
Charge Selectivity:
•Anionic (negatively charged) molecules: Repelled by the negatively charged glycocalyx, GBM proteoglycans, and slit diaphragm proteins; filtration restricted
•Neutral molecules: Filtered based on size alone
•Cationic (positively charged) molecules: More readily filtered than anionic molecules of similar size
Clinical Correlation:
•Normal urine contains <150 mg/day of protein (mostly Tamm-Horsfall protein from tubules, not filtered albumin)
•Glomerular proteinuria: Results from disruption of the filtration barrier
•Selective proteinuria: Primarily albumin (podocyte injury)
•Non-selective proteinuria: Albumin plus larger proteins (severe GBM damage)
•Loss of negative charge (e.g., in diabetes) precedes structural damage and contributes to early albuminuria
Glomerular Diseases and the Filtration Barrier
Damage to any component of the filtration barrier can result in proteinuria and glomerular disease:
Podocyte Injury:
•Minimal change disease (loss of foot processes, “effacement”)
•Focal segmental glomerulosclerosis (FSGS)
•Diabetic nephropathy (podocyte loss and foot process effacement)
•Genetic mutations (nephrin, podocin, α-actinin-4, TRPC6)
GBM Abnormalities:
•Alport syndrome (mutations in type IV collagen genes)
•Thin basement membrane disease
•Anti-GBM disease (Goodpasture syndrome)
Endothelial Injury:
•Thrombotic microangiopathies (HUS, TTP)
•Preeclampsia (endotheliosis)
Embryology and Development
Understanding renal embryology is essential for recognizing congenital anomalies and comprehending the developmental basis of kidney structure.
Three Successive Kidney Systems
During embryonic development, three successive kidney systems develop from the intermediate mesoderm along the nephrogenic cord:
1. Pronephros (Week 3-4)
•Most primitive and cranial kidney system
•Nonfunctional in humans
•Completely regresses by week 4
•Pronephric duct persists and becomes the mesonephric duct
2. Mesonephros (Week 4-8)
•Develops caudal to the pronephros
•Functions briefly as a primitive excretory organ in the embryo (weeks 6-10)
•Contains approximately 40 pairs of tubules that drain into the mesonephric (Wolffian) duct
•In males: Mesonephric duct and some tubules persist to form the epididymis, vas deferens, and seminal vesicles
•In females: Mesonephric structures largely degenerate
•Regresses as the metanephros develops
3. Metanephros (Week 5 onward)
•The definitive adult kidney
•Begins development in week 5 and becomes functional around week 9-10
•Develops from two sources:
•Ureteric bud (metanephric diverticulum): Outgrowth from the mesonephric duct that forms the collecting system
•Metanephric mesenchyme (metanephric blastema): Intermediate mesoderm that forms the nephrons
Development of the Metanephric Kidney
Ureteric Bud Derivatives (Collecting System): The ureteric bud undergoes repeated branching (15-20 generations) to form:
•Ureter
•Renal pelvis
•Major calyces (2-3)
•Minor calyces (8-12)
•Collecting ducts
•Papillary ducts
Metanephric Mesenchyme Derivatives (Nephrons): The metanephric mesenchyme, under induction by the ureteric bud, differentiates to form:
•Glomerulus
•Bowman capsule
•Proximal tubule
•Loop of Henle
•Distal tubule (up to the connecting tubule)
The distal tubule connects with the collecting duct to form a continuous tubular system from glomerulus to papilla.
Nephrogenesis Timeline
•Week 5: Ureteric bud and metanephric mesenchyme interact
•Week 8-9: First nephrons begin to form
•Week 10: Kidney begins producing urine (contributes to amniotic fluid)
•Week 20: Full complement of nephrons present in cortex
•Week 32-36: Nephrogenesis complete; no new nephrons form after birth
Critical Clinical Point:
•Nephrogenesis is complete by 32-36 weeks of gestation
•Premature infants born before 36 weeks may have reduced nephron numbers
•Postnatal kidney growth occurs through hypertrophy (increase in nephron size), not hyperplasia (increase in nephron number)
•Low nephron endowment (from prematurity, intrauterine growth restriction, maternal malnutrition, or genetic factors) predisposes to hypertension and chronic kidney disease in adulthood through hyperfiltration injury of remaining nephrons
Ascent of the Kidney
The metanephric kidney initially develops in the pelvis (at the level of S1-S2) and undergoes a relative “ascent” to its final position in the abdomen (T12-L3) due to differential growth of the embryo.
Process:
•Weeks 6-9: Kidney ascends from pelvis to lumbar region
•Blood supply changes during ascent: kidneys receive successive arterial branches from the aorta at progressively higher levels
•Lower temporary vessels normally degenerate
•Kidney rotates 90° medially during ascent, bringing the hilum to face medially
Anomalies of Ascent:
•Pelvic kidney: Kidney fails to ascend, remains in pelvis
•Horseshoe kidney: Fusion of lower poles (90%) or upper poles (10%) prevents normal ascent; kidneys caught by inferior mesenteric artery at L3-L5
Renal Innervation
The kidneys receive both sympathetic and sensory innervation, but lack significant parasympathetic innervation. Neural control plays important roles in regulating renal blood flow, renin release, and sodium reabsorption.
Sympathetic Innervation
Origin and Pathway:
•Spinal levels: T8-L1 (thoracolumbar)
•Preganglionic fibers: Synapse in the celiac and aorticorenal ganglia
•Postganglionic fibers: Travel along the renal artery and its branches to reach the kidney
Distribution:
•Afferent and efferent arterioles
•Juxtaglomerular cells (granular cells)
•Tubular epithelium (primarily thick ascending limb and distal convoluted tubule)
Receptor Types and Functions:
| Receptor | Location | Function |
| α1-Adrenergic | Afferent and efferent arterioles | Vasoconstriction → Decreased renal blood flow and GFR |
| β1-Adrenergic | Juxtaglomerular cells | Renin release → RAAS activation → Blood pressure elevation |
| α1 + β1 | Proximal tubule, thick ascending limb | Enhanced Na+ reabsorption → Volume retention |
Physiological Effects of Sympathetic Activation:
1.Renal vasoconstriction (decreased RBF and GFR)
2.Increased renin secretion (RAAS activation)
3.Increased tubular sodium reabsorption
4.Net effect: Sodium and water retention, increased blood pressure
Sensory Innervation
Pathway:
•Sensory nerve fibers travel with sympathetic nerves via the renal plexus
•Enter the spinal cord at T11-L2 levels
•Pain referred to flank, groin, and genitalia (dermatomes T11-L2)
Clinical Pearls:
•Nonobstructing renal calculi do NOT cause pain (kidney parenchyma has minimal pain innervation)
•Renal colic results from ureteral obstruction, not from the stone itself
•Pain from ureteral obstruction is caused by distension of the renal pelvis and ureter, transmitted via stretch receptors
•Referred pain patterns:
•Upper ureteral obstruction: Flank pain
•Mid-ureteral obstruction: Lower abdominal pain
•Lower ureteral obstruction: Groin and testicular/labial pain
Renal Denervation
Clinical Application: Renal denervation has emerged as a treatment for resistant hypertension (uncontrolled blood pressure despite ≥3 antihypertensive medications including a diuretic).
Method:
•Transcatheter radiofrequency or ultrasound ablation of renal sympathetic nerves in the adventitia of the renal arteries
Effects:
•Reduces renal vasoconstriction
•Decreases renin release
•Reduces tubular sodium reabsorption
•Lowers systemic blood pressure
FDA Approval:
•Approved for resistant hypertension in 2023
•Studies show average blood pressure reduction of 5-10 mmHg systolic
•Minimally invasive procedure with low complication rates
Contraindications:
•Renal artery stenosis
•GFR <40 mL/min/1.73m²
•Solitary functioning kidney
•Unsuitable renal artery anatomy
Anatomical Variants
Anatomical variations of the kidney and urinary tract are common and have significant clinical implications for diagnosis, surgical planning, and understanding complications.
1. Horseshoe Kidney
Prevalence: 1 in 400 individuals (most common fusion anomaly)
Anatomy:
•Fusion of the lower poles (90%) or upper poles (10%) across the midline
•Isthmus (connecting tissue) lies anterior to the aorta and IVC
•Arrested ascent: Kidneys caught by the inferior mesenteric artery at L3-L5 level
•Abnormal orientation: Renal pelves face anteriorly instead of medially
Associations:
•Turner syndrome (7% of patients)
•Trisomy 18 (20% of patients)
•VACTERL association (Vertebral, Anal, Cardiac, Tracheoesophageal, Renal, Limb anomalies)
Complications:
•Ureteropelvic junction (UPJ) obstruction (30%)
•Nephrolithiasis (increased risk due to impaired drainage)
•Recurrent urinary tract infections
•Increased risk of trauma (anterior position)
•Slightly increased risk of Wilms tumor and renal cell carcinoma
2. Accessory Renal Arteries
Prevalence: 20-30% of individuals
Anatomy:
•Additional renal arteries arising from the aorta or iliac arteries
•Most commonly supply the lower pole
•Are end arteries with no collateral circulation
•May cross anterior to the ureter
Clinical Significance:
•Must be preserved during surgery (nephrectomy, transplantation)
•Injury leads to segmental infarction
•Can cause UPJ obstruction if crossing anterior to the ureter (crossing vessel)
•Preoperative imaging essential: CT angiography or MR angiography required before donor nephrectomy or renal surgery to identify all vessels
3. Duplicated Collecting System
Prevalence: 1 in 125 individuals (most common upper urinary tract anomaly)
Types:
Complete Duplication:
•Two separate ureters from a single kidney draining separately into the bladder
•Follows the Weigert-Meyer rule:
•Upper pole ureter inserts medially and inferiorly (ectopic insertion or ureterocele) → prone to obstruction
•Lower pole ureter inserts laterally and superiorly (normal trigone position) → prone to vesicoureteral reflux (VUR)
Partial Duplication (Bifid Ureter):
•Two ureters that join before entering the bladder
•Usually asymptomatic
Complications:
•Recurrent urinary tract infections
•Hydronephrosis (upper pole system)
•Vesicoureteral reflux (lower pole system)
•Ureterocele (cystic dilation of distal ureter) with obstruction
4. Renal Ectopia
Prevalence: 1 in 900 individuals
Types:
Pelvic Kidney:
•Kidney fails to ascend, remains in the pelvis
•Often asymptomatic, discovered incidentally
•Increased risk of trauma, stones, infection
Crossed Ectopia:
•Kidney crosses the midline to the opposite side
•May be fused or unfused with the contralateral kidney
•More common: Left kidney crosses to right side
Thoracic Kidney:
•Rare: Kidney located above the diaphragm in the thorax
•May be mistaken for a posterior mediastinal mass on chest X-ray
Clinical Features:
•Often asymptomatic, incidental finding on imaging
•Abnormal vasculature: Multiple small arteries from nearby vessels
•Increased risk of stones, infection, and trauma
•May complicate surgical approach if undiagnosed
Clinical Pearl: Preoperative Imaging
Preoperative imaging (CT angiography or MR angiography) is essential before renal surgery or transplantation to identify anatomical variants. Failure to recognize accessory arteries or vascular anomalies can lead to segmental infarction, while undiagnosed horseshoe kidney or ectopia can complicate surgical approach and increase complication rates.
Summary
Advanced renal anatomy encompasses the macroscopic and microscopic structures essential for kidney function. The kidneys are paired retroperitoneal organs located at T12-L3, receiving 20-25% of cardiac output despite comprising only 0.5% of body weight. Each kidney contains 1-1.5 million nephrons (range 200,000-2.5 million), the functional units responsible for filtration, reabsorption, and secretion.
The internal architecture consists of the cortex (outer, vascular region containing glomeruli and convoluted tubules) and medulla (inner region containing loops of Henle and collecting ducts organized into 8-18 renal pyramids). The collecting system includes minor calyces, major calyces, renal pelvis, and ureter.
The nephron consists of the renal corpuscle (glomerulus and Bowman capsule) and renal tubule (proximal tubule, loop of Henle, distal tubule, and collecting duct). Cortical nephrons (85%) have short loops, while juxtamedullary nephrons (15%) have long loops extending into the medulla, essential for urine concentration.
The renal vasculature features a unique dual capillary system: glomerular capillaries for filtration and peritubular capillaries/vasa recta for reabsorption. The interlobar and arcuate arteries are end arteries; occlusion causes segmental infarction. The Brödel line is a relatively avascular plane used for percutaneous access. The left renal vein (7-10 cm) is longer than the right (2-4 cm), making the left kidney preferred for transplantation.
The juxtaglomerular apparatus (macula densa, granular cells, extraglomerular mesangial cells) regulates GFR through tubuloglomerular feedback and controls blood pressure through renin secretion and RAAS activation.
The glomerular filtration barrier consists of three layers: fenestrated endothelium, glomerular basement membrane, and podocyte foot processes with slit diaphragms. This barrier provides both size selectivity (restricting molecules >69 kDa) and charge selectivity (repelling negatively charged proteins). Podocyte injury is the most common cause of nephrotic-range proteinuria.
Nephrogenesis is complete by 32-36 weeks gestation; no new nephrons form after birth. Low nephron endowment (prematurity, intrauterine growth restriction) predisposes to hypertension and CKD through hyperfiltration injury.
Sympathetic innervation (T8-L1) controls renal vasoconstriction, renin release, and sodium reabsorption. Renal denervation is FDA-approved for resistant hypertension. Sensory innervation (T11-L2) mediates pain; nonobstructing renal calculi do not cause pain, but ureteral obstruction does (renal colic).
Common anatomical variants include horseshoe kidney (1:400), accessory renal arteries (20-30%), duplicated collecting system (1:125), and renal ectopia (1:900). Preoperative imaging is essential to identify variants and prevent complications during surgery.
Clinical Pearls
1.Renal Blood Flow: The kidneys receive 20-25% of cardiac output (~1,200 mL/min) despite comprising only 0.5% of body weight. This high flow enables filtration but increases vulnerability to ischemic injury and makes the kidneys susceptible to damage from hypotension, sepsis, and nephrotoxic drugs.
2.End Arteries: Interlobar and arcuate arteries are end arteries with no collateral circulation. Occlusion (from emboli, vasculitis, or surgical injury) causes segmental infarction. This is critical for surgical planning and understanding the wedge-shaped infarcts seen on imaging.
3.Brödel Line: The relatively avascular plane between anterior and posterior segmental arteries (slightly posterior to the lateral convex border) is the preferred entry point for percutaneous nephrolithotomy to minimize bleeding risk.
4.Renal Vein Length Asymmetry: The left renal vein (7-10 cm) is longer than the right (2-4 cm). The left kidney is preferred for living donor transplantation due to easier vascular anastomosis. Left varicocele is common and benign; isolated right varicocele is a red flag for malignancy (RCC with tumor thrombus or IVC obstruction).
5.Nephron Number Variation: Nephron number varies widely (200,000 to 2.5 million per kidney). Low nephron endowment (from prematurity, low birth weight, maternal malnutrition) increases lifelong risk of hypertension and CKD via hyperfiltration injury of remaining nephrons. Nephrogenesis is complete by 32-36 weeks; no new nephrons form after birth.
6.Juxtaglomerular Apparatus Function: The macula densa senses tubular NaCl concentration and regulates GFR through tubuloglomerular feedback (autoregulation) and blood pressure through RAAS activation. This is the target of ACE inhibitors and ARBs, cornerstone therapies for hypertension, heart failure, and CKD.
7.Glomerular Filtration Barrier: The three-layer barrier (fenestrated endothelium, GBM, podocytes) provides both size selectivity (restricts molecules >69 kDa) and charge selectivity (repels negatively charged proteins). Podocyte injury is the most common cause of nephrotic-range proteinuria (minimal change disease, FSGS, diabetic nephropathy).
8.Isolated Right Varicocele: Left varicoceles (85-90%) are common and benign due to left gonadal vein drainage into the left renal vein. Isolated right varicocele is a red flag for malignancy and warrants urgent imaging to exclude renal cell carcinoma with tumor thrombus or IVC obstruction.
9.Accessory Renal Arteries: Present in 20-30% of individuals, these are end arteries that must be preserved during surgery. Injury leads to segmental infarction. Preoperative CT or MR angiography is essential before donor nephrectomy or renal surgery to identify all vessels.
10.Nephrogenesis Complete by Birth: No new nephrons form after 32-36 weeks gestation. Postnatal kidney growth is via hypertrophy only. Early-life injury (prematurity, intrauterine growth restriction, nephrotoxic drugs) has permanent consequences for kidney function and increases risk of CKD and hypertension in adulthood.
Multiple Choice Questions
Basic Level (Medical Students and Residents)
Question 1: What percentage of cardiac output do the kidneys receive?
A) 5-10%
B) 10-15%
C) 20-25%
D) 30-35%
E) 40-45%
Answer: C) 20-25%
Explanation: The kidneys receive approximately 20-25% of cardiac output (about 1,200 mL/min), despite comprising only 0.5% of total body weight. This remarkably high blood flow is essential for their filtration function, as the kidneys filter approximately 180 liters of blood daily to produce 1-2 liters of urine. This high flow rate also makes the kidneys particularly vulnerable to ischemic injury during hypotension or shock.
Question 2: What is the correct anterior-to-posterior arrangement of structures at the renal hilum?
A) Artery, Vein, Pelvis
B) Vein, Artery, Pelvis
C) Pelvis, Artery, Vein
D) Vein, Pelvis, Artery
E) Artery, Pelvis, Vein
Answer: B) Vein, Artery, Pelvis
Explanation: The structures at the renal hilum are arranged from anterior to posterior as: Vein (most anterior), Artery (middle), Pelvis (most posterior). This can be remembered by the mnemonic “VAP.” Understanding this anatomical relationship is crucial during surgical dissection, vascular access procedures, and interpretation of cross-sectional imaging. The renal vein is the most superficial structure encountered during surgical approach to the hilum.
Question 3: Approximately how many nephrons does each adult kidney contain?
A) 100,000-500,000
B) 500,000-1 million
C) 1-1.5 million
D) 2-3 million
E) 5-10 million
Answer: C) 1-1.5 million
Explanation: Each adult kidney contains approximately 1 to 1.5 million nephrons, though there is considerable variation between individuals (range 200,000 to over 2.5 million). Importantly, nephrogenesis is complete by 32-36 weeks of gestation, and no new nephrons form after birth. Individuals born with fewer nephrons (due to prematurity, intrauterine growth restriction, or genetic factors) have increased susceptibility to hypertension and chronic kidney disease later in life through hyperfiltration injury of the remaining nephrons.
Question 4: What is the Brödel line?
A) The boundary between cortex and medulla
B) A relatively avascular plane between anterior and posterior segmental arteries
C) The line of fusion in a horseshoe kidney
D) The path of the ureter as it crosses the iliac vessels
E) The demarcation between upper and lower renal poles
Answer: B) A relatively avascular plane between anterior and posterior segmental arteries
Explanation: The Brödel line (also called the avascular plane of Brödel) is a relatively avascular longitudinal zone between the anterior and posterior segmental arterial territories, typically located slightly posterior to the lateral convex border of the kidney. This plane is the preferred entry point for percutaneous nephrolithotomy because it minimizes the risk of significant bleeding. The existence of this relatively avascular plane is due to the end-artery nature of the segmental renal arteries, which have minimal collateral circulation.
Question 5: How many layers comprise the glomerular filtration barrier?
A) One layer
B) Two layers
C) Three layers
D) Four layers
E) Five layers
Answer: C) Three layers
Explanation: The glomerular filtration barrier consists of three distinct layers: (1) fenestrated endothelium of the glomerular capillaries (with pores 70-100 nm in diameter), (2) glomerular basement membrane (GBM, 300-350 nm thick, composed of type IV collagen, laminin, and negatively charged proteoglycans), and (3) podocyte foot processes with slit diaphragms (25-60 nm gaps bridged by nephrin and other proteins). Together, these three layers provide both size selectivity (restricting molecules >69 kDa) and charge selectivity (repelling negatively charged proteins like albumin). Disruption of any layer can result in proteinuria.
Advanced Level (Nephrology Fellows and Nephrologists)
Question 6: Which of the following best describes the mechanism of tubuloglomerular feedback?
A) Increased NaCl delivery to the macula densa → adenosine release → afferent arteriolar dilation → increased GFR
B) Increased NaCl delivery to the macula densa → adenosine release → afferent arteriolar constriction → decreased GFR
C) Decreased NaCl delivery to the macula densa → renin release → efferent arteriolar constriction → increased GFR
D) Decreased NaCl delivery to the macula densa → prostaglandin release → afferent arteriolar dilation → increased GFR
E) Increased NaCl delivery to the macula densa → nitric oxide release → efferent arteriolar dilation → decreased GFR
Answer: B) Increased NaCl delivery to the macula densa → adenosine release → afferent arteriolar constriction → decreased GFR
Explanation: Tubuloglomerular feedback is a local negative feedback mechanism that maintains stable GFR despite fluctuations in systemic blood pressure. When GFR increases, more NaCl is delivered to the macula densa (specialized cells in the thick ascending limb of the loop of Henle). The macula densa senses this elevated NaCl concentration and releases ATP and adenosine. Adenosine causes vasoconstriction of the afferent arteriole, which decreases glomerular capillary pressure and returns GFR to baseline. This autoregulatory mechanism operates effectively when mean arterial pressure is between 80-180 mmHg. Understanding this mechanism is important for interpreting the effects of NSAIDs (which inhibit afferent arteriolar dilation) and ACE inhibitors (which prevent efferent arteriolar constriction) on GFR.
Question 7: A 35-year-old man presents with a newly discovered right-sided varicocele. What is the most important next step?
A) Reassurance that varicoceles are common and benign
B) Surgical ligation of the varicocele
C) Urgent imaging of the abdomen and retroperitoneum
D) Semen analysis
E) Observation and follow-up in 6 months
Answer: C) Urgent imaging of the abdomen and retroperitoneum
Explanation: Isolated right varicocele is a red flag for malignancy and warrants urgent imaging to exclude renal cell carcinoma with tumor thrombus or inferior vena cava obstruction. This is because of the anatomical asymmetry of gonadal vein drainage: the left gonadal vein drains into the left renal vein at a right angle (making left varicoceles common and usually benign, accounting for 85-90% of cases), while the right gonadal vein drains directly into the inferior vena cava at an acute angle. A right varicocele suggests obstruction of venous drainage, which can be caused by a renal tumor with tumor thrombus extending into the renal vein or IVC, or by extrinsic compression from a retroperitoneal mass. Urgent CT or MRI of the abdomen and pelvis is indicated to rule out malignancy.
Question 8: A patient with horseshoe kidney is at increased risk for all of the following EXCEPT:
A) Ureteropelvic junction obstruction
B) Nephrolithiasis
C) Wilms tumor
D) Renal cell carcinoma
E) Polycystic kidney disease
Answer: E) Polycystic kidney disease
Explanation: Horseshoe kidney (prevalence 1 in 400) is associated with increased risk of ureteropelvic junction obstruction (30% of cases, due to high insertion of the ureter and abnormal orientation), nephrolithiasis (due to impaired drainage), recurrent UTIs, increased risk of trauma (due to anterior position), and slightly increased risk of Wilms tumor and renal cell carcinoma. However, horseshoe kidney is NOT associated with polycystic kidney disease. Horseshoe kidney is associated with certain genetic syndromes including Turner syndrome (7% of patients), Trisomy 18 (20% of patients), and VACTERL association. The isthmus connecting the kidneys lies anterior to the aorta and IVC, and the kidneys are typically caught by the inferior mesenteric artery at L3-L5, preventing normal ascent.
Question 9: Renal denervation for resistant hypertension works primarily through which mechanism?
A) Increasing renin release from juxtaglomerular cells
B) Enhancing sodium reabsorption in the proximal tubule
C) Reducing sympathetic-mediated renal vasoconstriction and renin release
D) Stimulating prostaglandin production in the macula densa
E) Blocking angiotensin II receptors in the efferent arteriole
Answer: C) Reducing sympathetic-mediated renal vasoconstriction and renin release
Explanation: Renal denervation involves transcatheter radiofrequency or ultrasound ablation of renal sympathetic nerves in the adventitia of the renal arteries. This procedure reduces sympathetic innervation to the kidneys, which normally acts via α1-adrenergic receptors (causing renal vasoconstriction) and β1-adrenergic receptors (stimulating renin release from juxtaglomerular cells and enhancing tubular sodium reabsorption). By ablating these sympathetic nerves, renal denervation reduces renal vasoconstriction, decreases renin release (reducing RAAS activation), and reduces tubular sodium reabsorption, all of which contribute to lowering blood pressure. The procedure is FDA-approved for resistant hypertension (uncontrolled BP despite ≥3 antihypertensive medications including a diuretic) and typically reduces systolic blood pressure by 5-10 mmHg.
Question 10: In a patient with a duplicated collecting system, the Weigert-Meyer rule states that:
A) The upper pole ureter inserts laterally and superiorly, predisposing to reflux
B) The lower pole ureter inserts medially and inferiorly, predisposing to obstruction
C) The upper pole ureter inserts medially and inferiorly, predisposing to obstruction
D) Both ureters insert at the same location in the bladder
E) The upper pole ureter always drains into the lower pole ureter
Answer: C) The upper pole ureter inserts medially and inferiorly, predisposing to obstruction
Explanation: The Weigert-Meyer rule describes the relationship between ureteral insertion points and associated complications in complete ureteral duplication. The rule states that the upper pole ureter inserts medially and inferiorly (ectopic insertion, often into the bladder neck, urethra, or vagina in females, or may form a ureterocele), which predisposes to obstruction. In contrast, the lower pole ureter inserts laterally and superiorly at the normal trigone position, but this lateral insertion predisposes to vesicoureteral reflux (VUR) due to a shorter intramural tunnel and inadequate valve mechanism. Understanding this rule is essential for diagnosing and managing complications of duplicated collecting systems, which occur in approximately 1 in 125 individuals and represent the most common upper urinary tract anomaly.
References
1.National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK). “Your Kidneys & How They Work.” https://www.niddk.nih.gov/health-information/kidney-disease/kidneys-how-they-work
2.Blausen.com staff (2014). “Medical gallery of Blausen Medical 2014”. WikiJournal of Medicine 1(2). DOI:10.15347/wjm/2014.010. https://commons.wikimedia.org/wiki/File:Blausen_0592_KidneyAnatomy_01.png
3.OpenStax College. Anatomy & Physiology, Connexions Web site. http://cnx.org/content/col11496/1.6/, Jun 19, 2013. https://commons.wikimedia.org/wiki/File:2610_The_Kidney.jpg
4.M•Komorniczak (Wikimedia Commons). “Renal corpuscle-en” (derivative work). https://en.wikipedia.org/wiki/Podocyte
5.StatPearls Publishing. “Anatomy, Abdomen and Pelvis, Kidneys.” https://www.ncbi.nlm.nih.gov/books/NBK482385/
6.KDIGO (Kidney Disease: Improving Global Outcomes). Clinical Practice Guidelines. https://kdigo.org/guidelines/
Copyright: All content © Kidney-Hub. All images used under Creative Commons licenses with proper attribution.
This educational material is provided for medical education purposes.